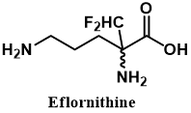
The drug Eflornithine (also called Vaniqa or α-difluoromethylornithine) is a selective inhibitor of the enzyme ornithine decarboxylase (ODC). It is useful in the treatment of African sleeping sickness and excessive hair growth. The clinical importance of this drug is indicated by its presence on the WHO’s list of most essential medicines for healthcare systems [1]. In this article I examine the biochemical and molecular aspects of Eflornithine's mechanism of action.
African sleeping sickness
African sleeping sickness (trypanosomiasis) is caused by the parasitic protozoa Trpanosoma brucei, usually spread by the bite of the tsetse fly. The disease progression is divided into two stages [2]. In the first, the parasite replicates in the host’s blood, lymph, and subcutaneous tissues. The disease progresses to the second stage when the parasite crosses the blood-brain barrier and infects the central nervous system.
Eflornithine is used to treat second stage cases of African sleeping sickness. It acts by irreversibly inhibiting the enzyme ornithine decarboxylase (ODC), an enzyme which converts ornithine to putrescine.
Eflornithine is used to treat second stage cases of African sleeping sickness. It acts by irreversibly inhibiting the enzyme ornithine decarboxylase (ODC), an enzyme which converts ornithine to putrescine.
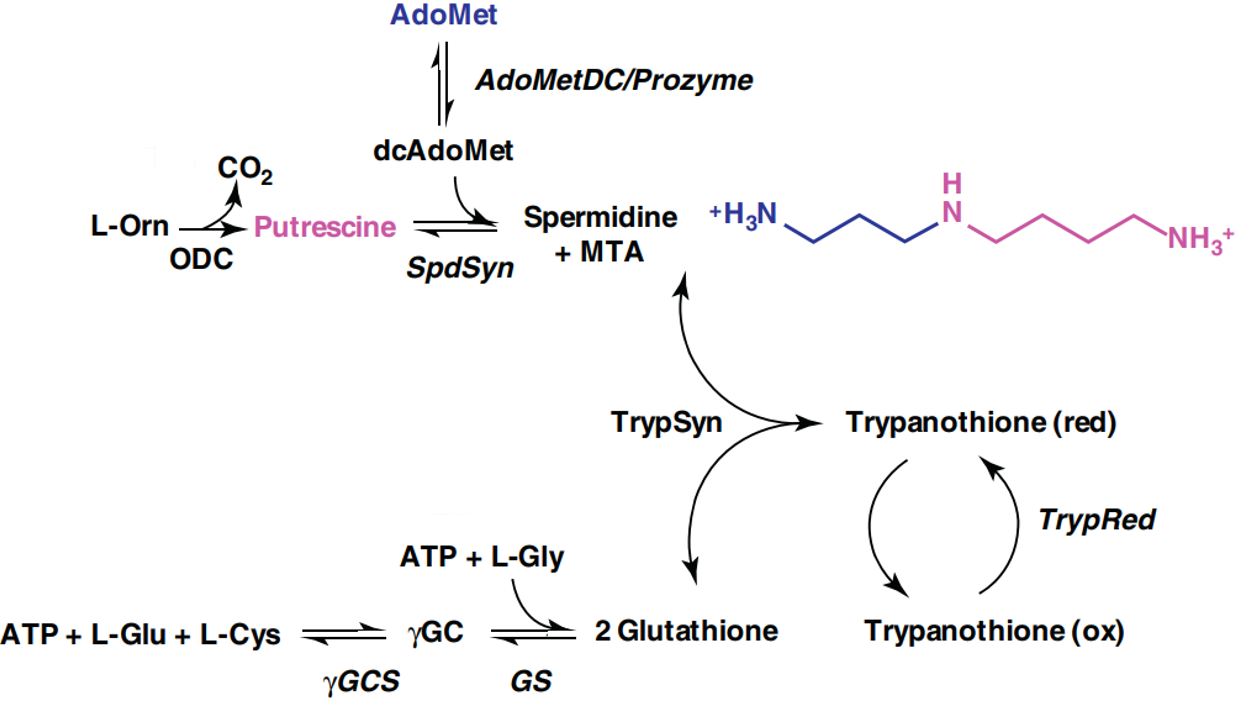
This is the rate-limiting step in the biosynthesis of polyamines [3]. Polyamines are cationic small molecules required broadly for growth and proliferation in eukaryotic cells and bacteria. The periodic positive charges of these compounds allow them to interact with negatively charged sites on biomolecules, including nucleic acids, lipids, and proteins. The metabolic pathway for polyamine synthesis in T. brucei is shown below [3].
The biological functions of polyamines are diverse. They regulate gene expression through modifying chromatin structure and post-transcriptional mechanisms [4]. In T. brucei, the polyamine spermidine is also a required precursor to trypanothione. This unusual dimer of glutathione is a central component of the parasite’s defense against oxidative stress [5]. Reactive oxygen species such as peroxides are enzymatically reduced with concomitant oxidation of trypanothione. T. brucei is particularly dependent on this enzyme system since it is one of the rare species which lacks catalase, a common peroxide-decomposing enzyme.
While polyamines are present in the host environment, T. brucei is inefficient at importing them. Therefore, this pathogen is highly dependent on polyamine biosynthesis [6]. Overall, these properties make polyamine synthesis an excellent therapeutic target. Nevertheless, it is worth remembering that human cells also require and manufacture polyamines through a similar pathway. This raises concern over possible toxicity, but fortunately human cells prove much less susceptible to eflornithine. This is, in part, due to a faster turnover rate of ODC in humans [7].
While polyamines are present in the host environment, T. brucei is inefficient at importing them. Therefore, this pathogen is highly dependent on polyamine biosynthesis [6]. Overall, these properties make polyamine synthesis an excellent therapeutic target. Nevertheless, it is worth remembering that human cells also require and manufacture polyamines through a similar pathway. This raises concern over possible toxicity, but fortunately human cells prove much less susceptible to eflornithine. This is, in part, due to a faster turnover rate of ODC in humans [7].
Excessive hair growth
Eflornithine was originally developed as a treatment for African sleeping sickness. However, more recently it has also been widely adopted as a treatment for excessive/unwanted hair growth (hirsutism). The drug treats this ailment through the same mechanism: inhibiting ornithine decarboxylase and reducing polyamine production. Polyamine production is essential for hair growth. In fact, outer cells in growing hair follicles have high levels of ODC expression [8]. Furthermore, the polyamine spermidine promotes hair growth in human hair follicles [9]. Topical application of eflornithine is able to effectively inhibit polyamine synthesis and therefore reduce unwanted hair growth [10].
Eflornithine - an irreversible, mechanism-based inhibitor
Eflornithine is a mechanism-based inhibitor (also called suicide inhibitor). This interesting class of inhibitor inactivates its target enzyme by irreversible formation of a covalent bond. However, this mode of inhibition is distinct from general covalent enzyme inhibitors. They are initially unreactive, bearing a “masked” electrophile. To covalently modify the target, mechanism-based inhibitors must be accepted by the target enzyme as a substrate. The inhibitor binds the active site in a similar manner to substrate, and undergoes analogous chemistry catalyzed by the enzyme. The enzymatic modification chemically unveils the inhibitor's reactive group. Subsequent reaction with an enzymatic nucleophile covalently modifies the enzyme, irreversibly blocking its active site.
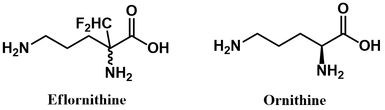
Another more colourful name given to this inhibitor class is “Trojan horse substrates.” Like in the classic Greek tale, the enzyme is deceived by the inhibitor, mistaking it for substrate. It permits the inhibitor entry into its active site and activates it, bringing about the enzyme's demise. Note the structural similarity between eflornithine and ornithine (ODC’s substrate).
Another more colourful name given to this inhibitor class is “Trojan horse substrates.” Like in the classic Greek tale, the enzyme is deceived by the inhibitor, mistaking it for substrate. It permits the inhibitor entry into its active site and activates it, bringing about the enzyme's demise. Note the structural similarity between eflornithine and ornithine (ODC’s substrate).
While often challenging to design, mechanism-based inhibitors often have significant advantages over other therapeutic inhibitor approaches. Since a single inhibitor molecule is enough to permanently inactivate an enzyme, this strategy affords excellent potency. Furthermore, the masked nature of the inhibitor’s reactive warhead offers superior selectivity relative to other covalent inhibitor strategies.
Eflornithine requires activation by ornithine decarboxylase, mechanistically analogous to substrate turnover, so we'll first review this enzyme’s catalytic mechanism.
Eflornithine requires activation by ornithine decarboxylase, mechanistically analogous to substrate turnover, so we'll first review this enzyme’s catalytic mechanism.
Mechanism of ornithine decarboxylase
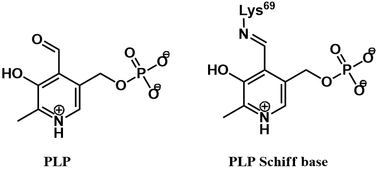
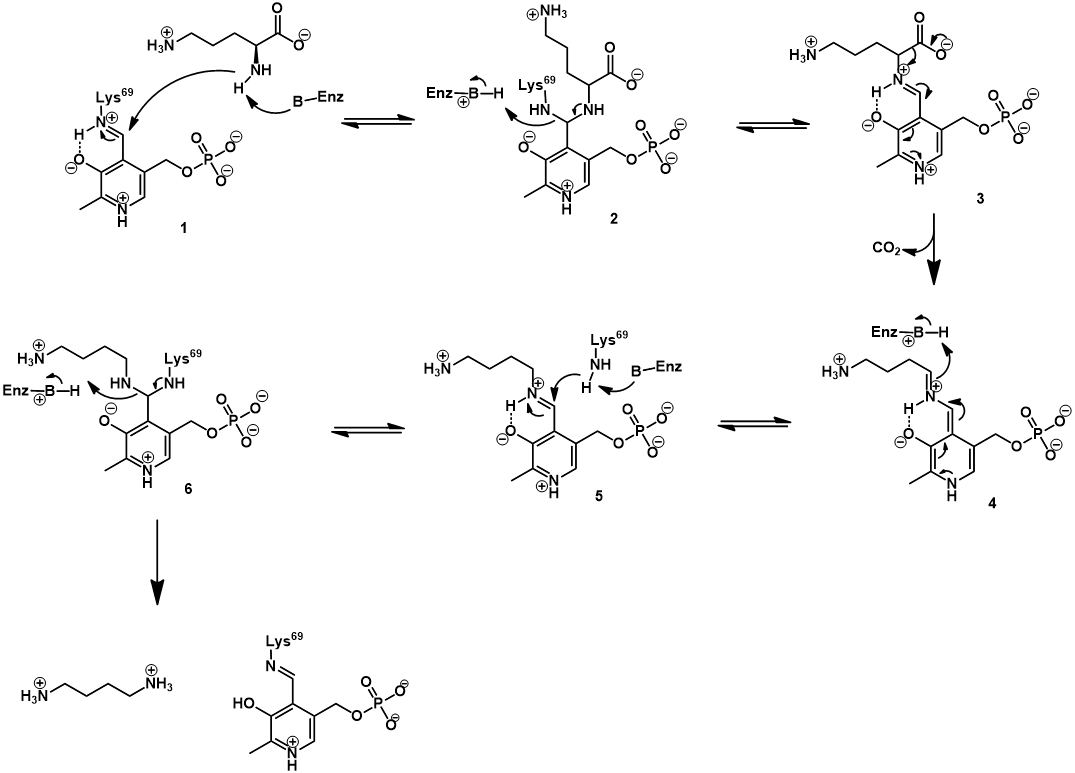
ODC uses pyridoxal phosphate (PLP, vitamin B6) as a cofactor. This cofactor is covalently bound to the enzyme active site via a Schiff base (also called an aldimine).
The PLP cofactor is essential for catalysis, activating the ornithine substrate. This is achieved through formation of a Schiff base between ornithine and PLP. Transfer of the PLP group occurs via Schiff base exchange (transamination). Mechanistically, this begins with nucleophilic attack of ornithine’s α-amine on the Schiff base of PLP and the active site lysine’s ε-amine (1), giving an aminoacetal intermediate (2). Collapse of this intermediate occurs with expulsion of the active site lysine, giving the Schiff base of ornithine and PLP (3). The PLP cofactor promotes decarboxylation by acting as an electron sink, stabilizing the carbanion character of the quinoid intermediate (4). Protonation of this intermediate gives the Schiff base of putrescine with PLP (5). Lastly, Schiff base exchange occurs once more, by nucleophilic attack of the active site lysine’s ε-amine (5). Collapse of the aminoacetal intermediate (6) releases the putrescine product and regenerates the enzyme-PLP Schiff base.
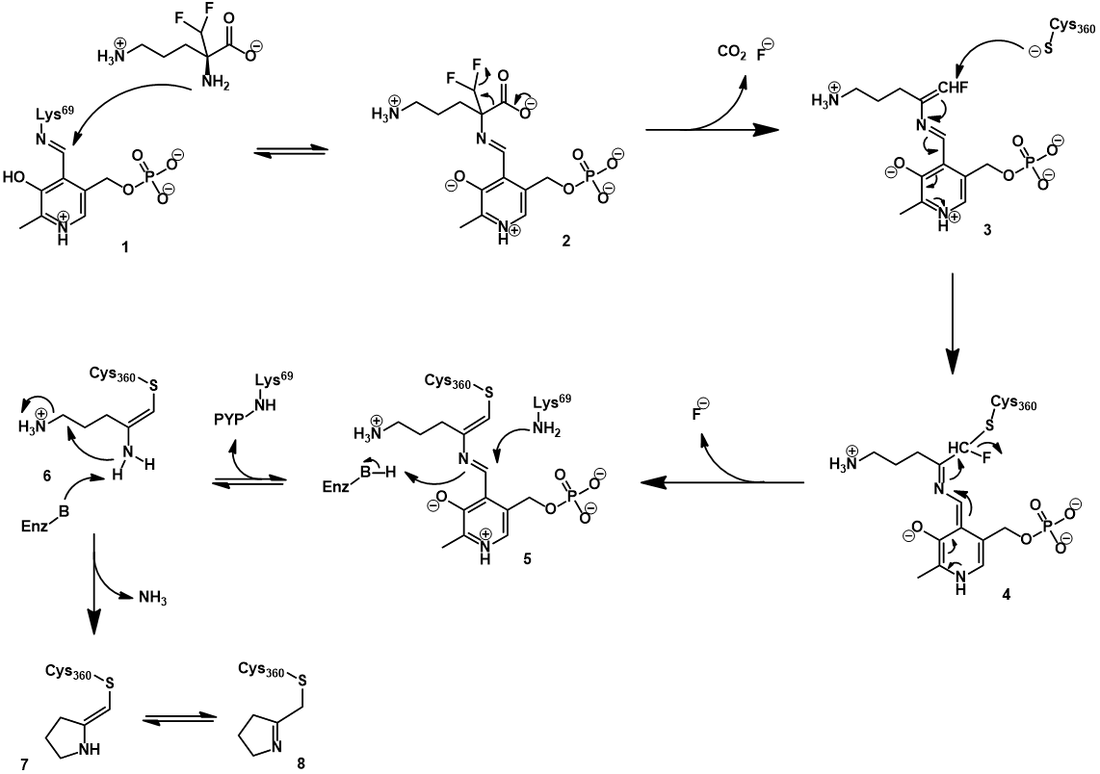
The mechanism of ODC inhibition by eflornithine
Elfornithine, being highly structurally similar to ornithine, binds to the active site of ODC in similar orientation. Its inhibitory mechanism begins in a manner analogous to ornithine decarboxylation. First, it forms a Schiff base with the PLP cofactor through Schiff base exchange (2). As before, this occurs through nucleophilic attack of eflornithine’s α-amine. Elfornithine is now highly activated for decarboxylation, with additional electronic stabilization afforded by the electron-withdrawing difluoro group. However, distinct from ornithine, eflornithine decarboxylation is followed by fluoride elimination, giving a highly electrophilic conjugated imine (3). This reactive moiety is subject to nucleophilic attack by the thiol of a nearby active site cysteine, forming a covalent bond (4). Elimination of the second fluoride occurs next, generating a second conjugated imine (5). Schiff base exchange can transfer the PLP back to the active site lysine, but the inhibitor remains irreversibly attached to the active site cysteine (6). Eventually, the bound inhibitor cyclizes with loss of an ammonium (7). This mechanism is supported by both mass spectrometry-based and spectrophotometric evidence [11]. With the active site permanently blocked by inhibitor, the enzyme is rendered inactive.

 RSS Feed
RSS Feed