In biochemistry and pharmacology, a variety of parameters are reported as measures of the potency of enzyme inhibitors/drugs, including Ki, Kd, IC50, and EC50. Though related, their definitions greatly differ. In this article, I highlight the meaning and significance of each term, with emphasis on their disambiguation and appropriate usage.
Ki vs Kd
Ki refers to inhibition constant, while Kd means dissociation constant. Both terms are used to describe the binding affinity that a small molecule or macromolecule has for an enzyme or receptor. The difference is that Kd is a more general, all-encompassing term. As discussed in my article on the difference between Km and Kd, Kd measures the equilibrium between the ligand-protein complex and the dissociated components.
Where [P] is the free protein concentration, [L] is the free ligand concentration, [PL] is the protein-ligand complex, k-1 is the dissociation rate constant for the complex and k1 is the association rate constant.
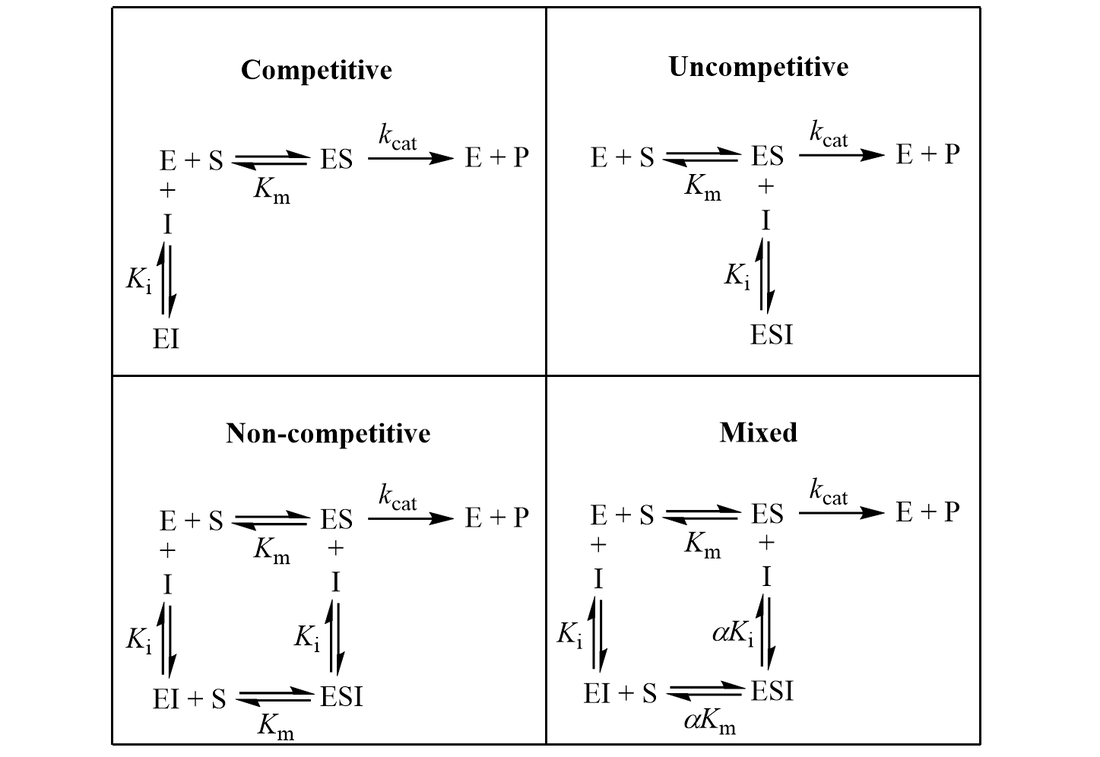
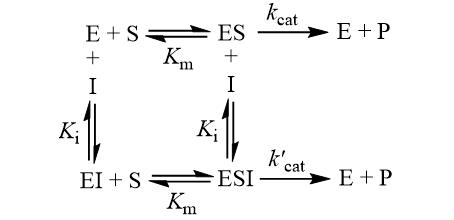
The Ki inhibition constant also represents a dissociation constant, but more narrowly for the binding of an inhibitor to an enzyme. That is, a ligand whose binding reduces the catalytic activity of the enzyme. The binding equilibrium described by the Ki value depends on the kinetic mechanism of inhibition. Common options include competitive, uncompetitive, non-competitive, and mixed inhibition. I define the equilibria for each below.
The Ki inhibition constant also represents a dissociation constant, but more narrowly for the binding of an inhibitor to an enzyme. That is, a ligand whose binding reduces the catalytic activity of the enzyme. The binding equilibrium described by the Ki value depends on the kinetic mechanism of inhibition. Common options include competitive, uncompetitive, non-competitive, and mixed inhibition. I define the equilibria for each below.
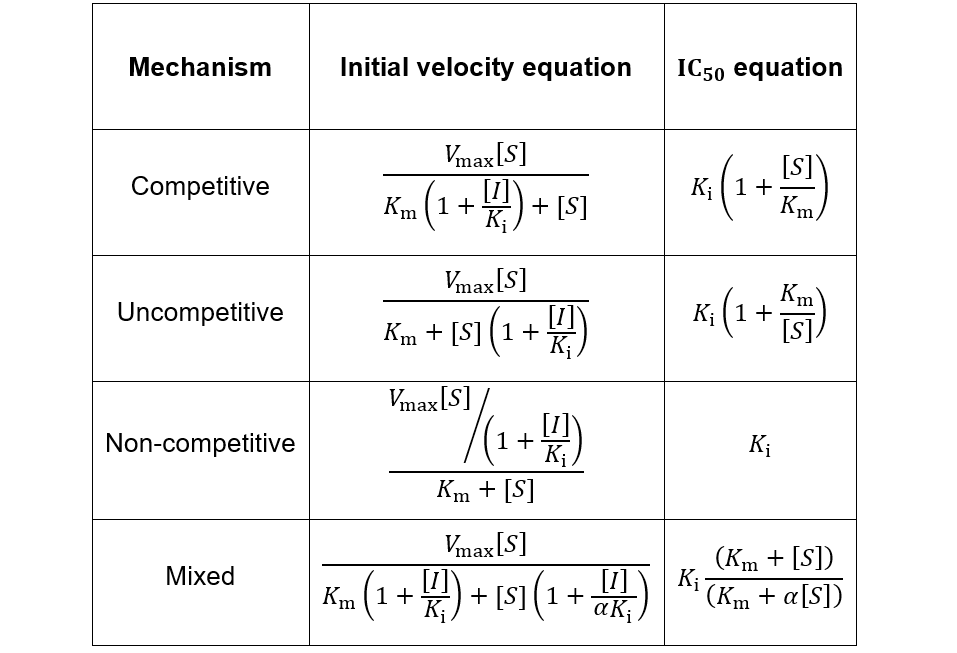
In competitive inhibition, the inhibitor binds only to free enzyme (E), not to the enzyme-substrate complex (ES). In uncompetitive inhibition, the inhibitor binds only to the enzyme-substrate complex. Mixed inhibition involves inhibitor binding to both free enzyme and enzyme-substrate complex with different binding constants (Ki and αKi). Lastly, non-competitive inhibition is a special case of mixed inhibition where substrate binding has no effect on inhibitor binding (α = 1).
Importantly, Ki values only accurately report a binding constant when the kinetic mechanism is correctly identified. The term ‘Ki’ is used whenever this binding constant is measured through inhibition kinetics, while ‘Kd’ is preferred when the binding is measured more directly (e.g. by fluorescence quenching, isothermal titration calorimetry, or surface plasmon resonance).
Importantly, Ki values only accurately report a binding constant when the kinetic mechanism is correctly identified. The term ‘Ki’ is used whenever this binding constant is measured through inhibition kinetics, while ‘Kd’ is preferred when the binding is measured more directly (e.g. by fluorescence quenching, isothermal titration calorimetry, or surface plasmon resonance).
IC50 vs Ki
IC50 stands for inhibitory concentration 50%. That is, the concentration of inhibitor required to reduce the biological activity of interest to half of the uninhibited value. Because it does not directly measure a binding equilibrium, IC50 is less precise than Ki or Kd. Importantly, the values obtained are highly dependent on the measurement conditions and the mechanism of inhibition. The simplicity and lack of assumptions required to generate these data make them useful. They are particularly convenient for characterizing the in vivo activity of a drug/inhibitor, where the various factors contributing to its potency cannot easily be independently accessed. For instance, the drug's in vivo distribution, cell wall permeability, target engagement, and clearance rate are all contributing factors. To measure IC50 values, dose-response curves are measured and fit to the following equation:
Where R is the response (fraction of the biological activity that has been inhibited), [I] is the inhibitor concentration and n is the Hill coefficient (a parameter which describes how steep the curve is).
IC50 values are also used for quick comparison of the in vitro activity of enzyme inhibitors. Despite their ease of measurement, this use case carries certain limitations and caveats. Meaningful comparison of IC50 values can be challenging, especially when an inhibitor’s mechanism is unknown or the experimental details are not reported in sufficient detail. IC50 values are highly dependent on the substrate concentration, with the nature of this dependence being determined by the inhibition mechanism. To demonstrate this, I show how IC50 and Ki are related for related for the common inhibition types.
First, I review how IC50 equations are derived from initial velocity equations, taking competitive inhibition as an example. An enzymatic reaction’s initial velocity under competitive inhibition (vi) is given by:
IC50 values are also used for quick comparison of the in vitro activity of enzyme inhibitors. Despite their ease of measurement, this use case carries certain limitations and caveats. Meaningful comparison of IC50 values can be challenging, especially when an inhibitor’s mechanism is unknown or the experimental details are not reported in sufficient detail. IC50 values are highly dependent on the substrate concentration, with the nature of this dependence being determined by the inhibition mechanism. To demonstrate this, I show how IC50 and Ki are related for related for the common inhibition types.
First, I review how IC50 equations are derived from initial velocity equations, taking competitive inhibition as an example. An enzymatic reaction’s initial velocity under competitive inhibition (vi) is given by:
Where Vmax is the maximum velocity ([E]T*kcat), [S] is the substrate concentration, and Km is the Michaelis constant. Without any competitive inhibitor, the initial velocity (v0) simplifies to typical Michaelis-Menten kinetics:
The IC50 concentration is reached when the ratio of the inhibited to the uninhibited reaction rate is 50%:
Solving for IC50, we obtain:
Using this strategy, it is possible to derive IC50 equations for any kinetic scheme of enzyme inhibition. I show the results of this for the common types of enzyme inhibition in the table below.
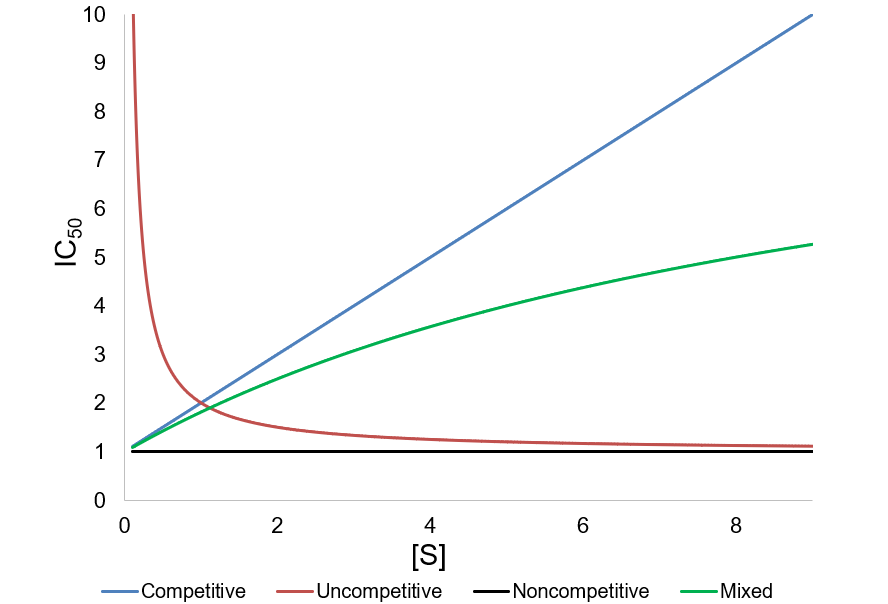
To view these relations graphically, I plot them supposing Km = Ki = 1 and α = 0.1:
Interestingly, non-competitive inhibition yields an IC50 value independent of substrate concentration. However, competitive inhibition IC50 values increase with increasing substrate concentration, while uncompetitive and mixed inhibition IC50 values decrease. Based on these dependencies, it is important to consider the substrate concentration and likely mechanism of inhibition when interpreting IC50 values. Where possible, this information should be reported alongside such data.
As a final caveat, another complication with IC50 values comes in the case of “tight binding” inhibitors, where the enzyme concentration affects the apparent value. This problem arises when the formation of enzyme-inhibitor complex appreciably depletes the concentration of free inhibitor (specifically, when Ki < [E]T). In these situations, a correction can be applied to account for the effect of this on the IC50 value.
As a final caveat, another complication with IC50 values comes in the case of “tight binding” inhibitors, where the enzyme concentration affects the apparent value. This problem arises when the formation of enzyme-inhibitor complex appreciably depletes the concentration of free inhibitor (specifically, when Ki < [E]T). In these situations, a correction can be applied to account for the effect of this on the IC50 value.
IC50 vs EC50
I conclude with a discussion of EC50, effective concentration 50%. This refers to the concentration of drug at which 50% of its maximum effect is achieved. The term is quite general, applying regardless of whether the drug enhances or diminishes the biological parameter. Its equation is a modified version of the IC50 equation, taking the ratio of the response (R) to the maximum response (Rmax):
In cases where a drug completely inhibits a biological activity at high dose, the values of EC50 and IC50 are identical (where Rmax = 100%). However, some drugs give only partial attenuation of a biological activity, even at high concentrations. In this case, IC50 values may be misleading about the potency of the drug. For instance, suppose an inhibitor reduces an enzyme’s activity to, at most, 60%. Here the IC50 value would be undefined, as 50% inhibition is never reached. Using EC50 values allows the dose-response of such drugs to be quantified and reported. In pharmacology, the concepts of drug potency and efficacy are worth examining in relation to IC50 and EC50 values. Potency refers to how much drug it takes to have maximum biological effect, while efficacy describes how large the effect size is. Therefore, potent drugs have low EC50 values but not necessarily low IC50 values. Furthermore, efficacious drugs have more similar IC50 and EC50 values than less efficacious drugs.
EC50 values are commonly used for in vivo measurements made on cells or animals; incomplete inhibition of a phenotype is often seen in complex biological systems. In principle though, this nomenclature can also be used for comparing the in vitro activity of partial enzyme inhibitors. As an example, consider a partial non-competitive inhibitor.
EC50 values are commonly used for in vivo measurements made on cells or animals; incomplete inhibition of a phenotype is often seen in complex biological systems. In principle though, this nomenclature can also be used for comparing the in vitro activity of partial enzyme inhibitors. As an example, consider a partial non-competitive inhibitor.
In this inhibition mechanism, binding of the inhibitor results in a decreased, but non-zero turnover rate of enzyme-substrate complex (0 < k’cat < kcat). To illustrate the difference between EC50 and IC50, I derive equations for both. For this kinetic scheme, the ratio of the inhibited to the uninhibited initial rate is:
The IC50 value is the concentration at which this ratio is reduced to 50%:
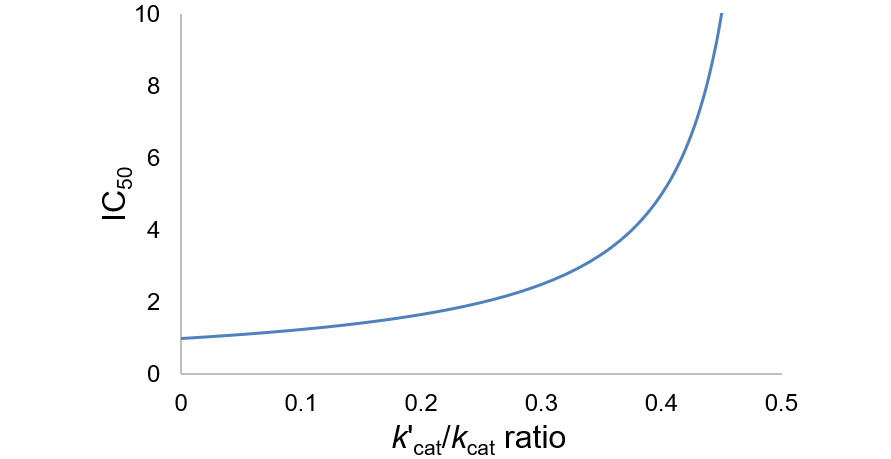
IC50 is not only a function of inhibitor binding affinity (Ki), but also on the relative amount of residual turnover rate of the ESI complex (k’cat). To visually illustrate this, I plot the IC50 equation assuming Ki = 1:
Progressively higher residual activity of the ESI complex (k’cat/kcat ratio) increases the IC50 value. It approaches infinity toward 50% residual activity, above which IC50 is undefined.
In contrast to IC50 values, EC50 reports on the binding affinity of the inhibitor, regardless of its efficacy. The fraction of enzyme (f) bound by inhibitor is given by:
In contrast to IC50 values, EC50 reports on the binding affinity of the inhibitor, regardless of its efficacy. The fraction of enzyme (f) bound by inhibitor is given by:
The effective concentration 50% is achieved when half of the enzyme is bound:
Though I’ve only derived equations for the non-competitive partial inhibition case, these trends hold for other inhibition mechanisms. The EC50 values are useful for comparing the relative binding affinities of partial enzyme inhibitors. Furthermore, EC50s are essential where IC50 values can’t be reported due to >50% of the biological activity being retained at maximum response. The main limitation of this parameter is that it offers no information about the efficacy of the drug/inhibitor. Overall, Kd, Ki, IC50, and EC50 values all have important roles in characterizing the biological activity of drugs and enzyme inhibitors. I hope that their differences, uses, and advantages/disadvantages are now clear.
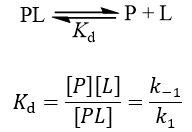
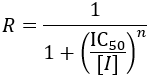
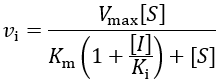
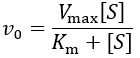
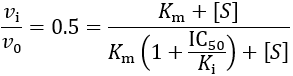
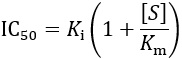
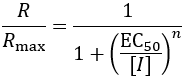
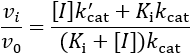
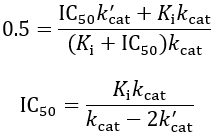
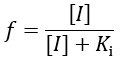
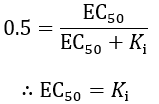
 RSS Feed
RSS Feed