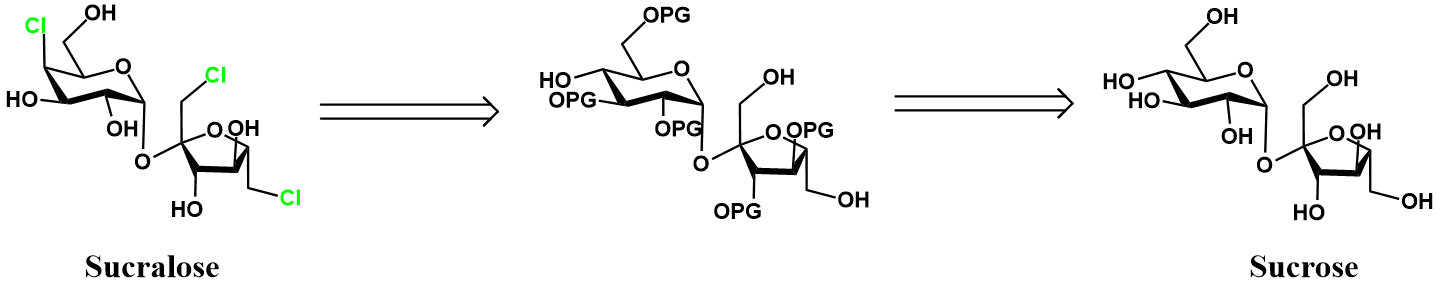
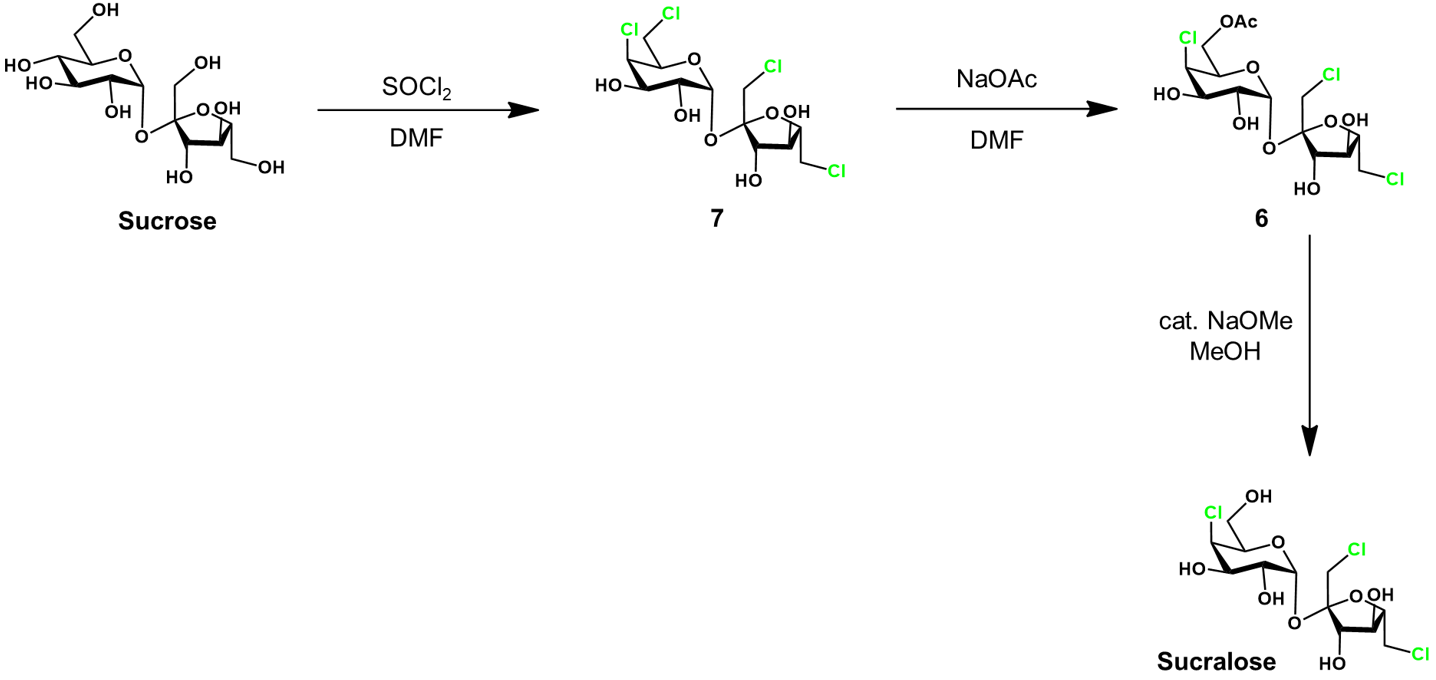
Control of chlorination by protecting groups [2]
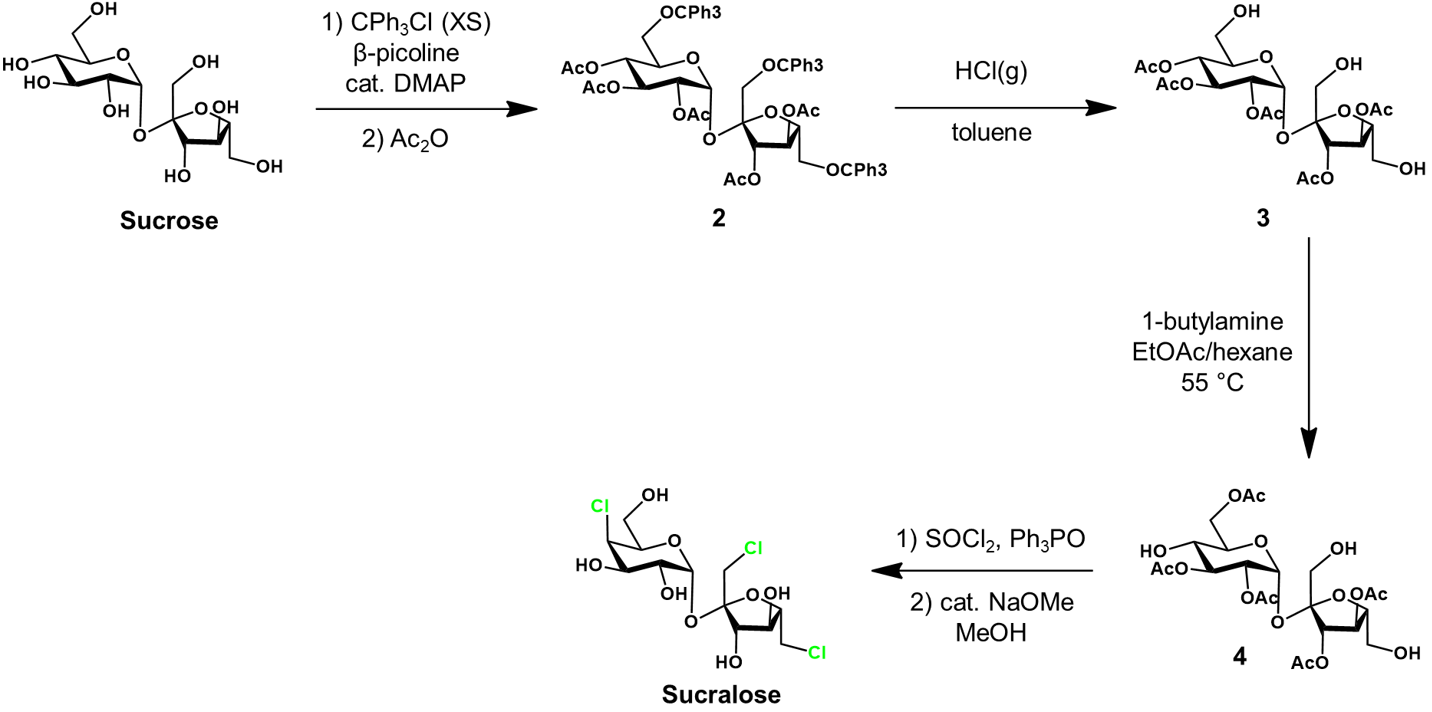
Next, the trityl groups are selectively removed by treatment with HCl in toluene, yielding 3. To attain the desired protecting group arrangement, the next step is critical. We require protection at glucose C6 but not C4. This is achieved by allowing acetyl migration from C4 to C6, giving protected intermediate 4. This transformation occurs with high selectively under treatment with 1-butylamine in a mixture of ethyl acetate and hexane.
Protected intermediate 4 is chlorinated with thionyl chloride (SOCl2) and triphenylphosphine oxide (Ph3PO). The thionyl chloride first chlorinates the triphenylphosphine oxide, generating the active chlorinating agent. This condition is gentler and reduces side reactions/product decomposition compared to using thionyl chloride alone. Lastly, the acetyl groups are deprotected under basic conditions in methanol to afford sucralose. Sucralose is conveniently purified by recrystallization from methanol/water.
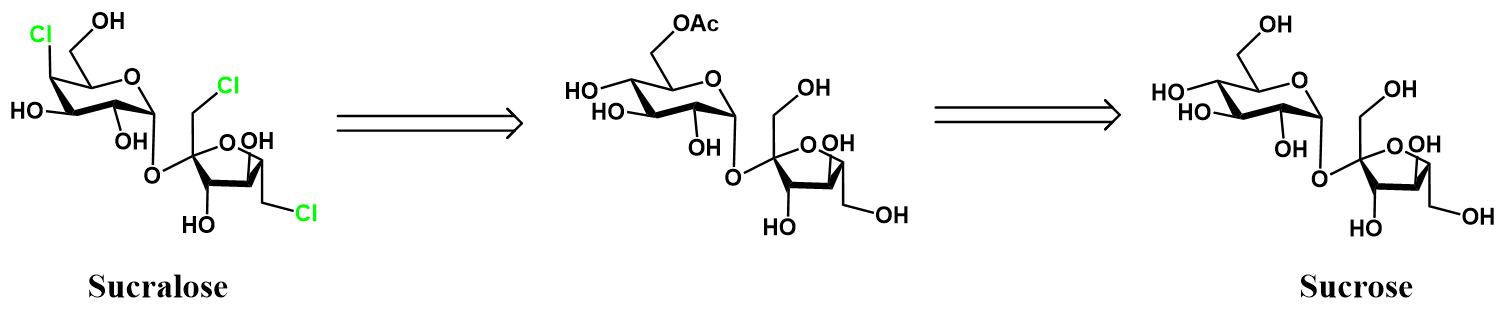
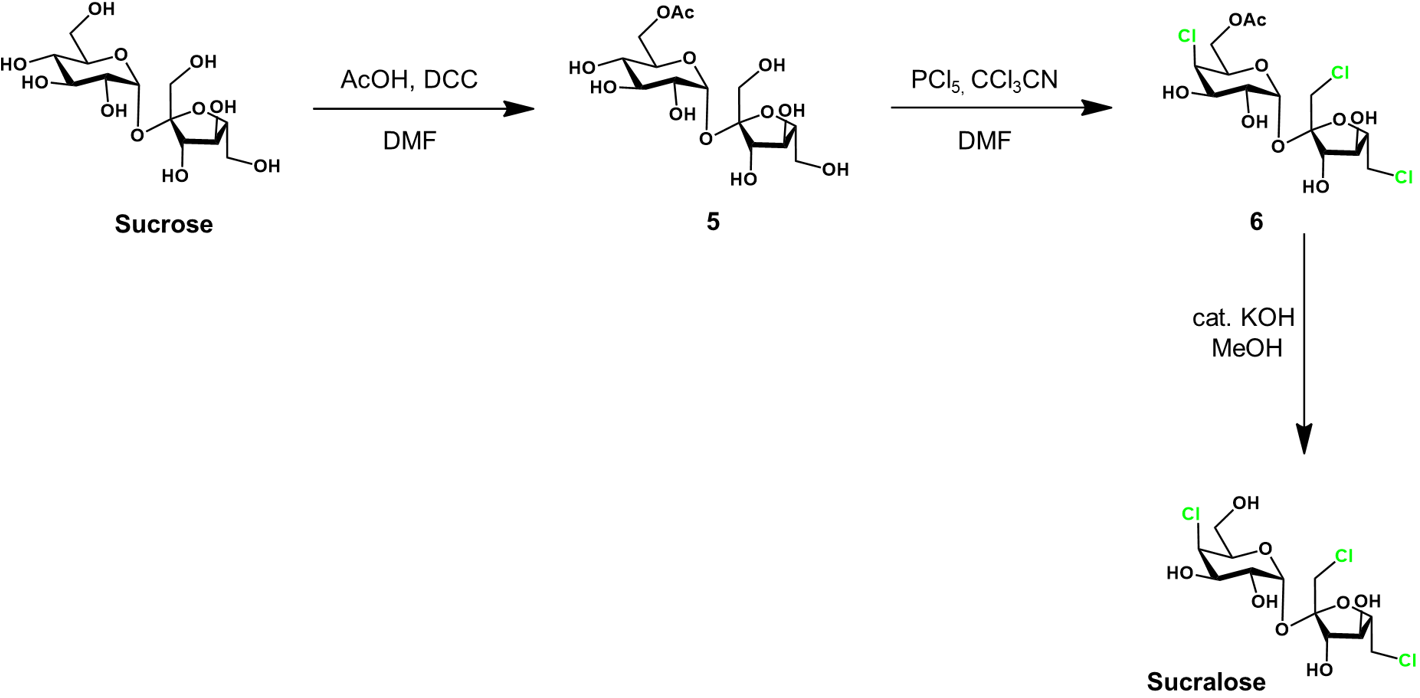
Selective chlorination of sucrose-6-acetate [3]
The selective chlorination of 5 to 6 is achieved using phosphorous pentachloride (PCl5) as the chlorinating agent. Trichloroacetontrile (CCl3CN) is used as an additive in the reaction, dramatically increasing the reaction rate through an unclarified mechanism. The selectivity attained in this reaction is impressive, likely requiring the carefully optimized conditions. Lastly, the sucralose-6-acetate 6 is deprotected in basic methanol to yield sucralose.
 RSS Feed
RSS Feed