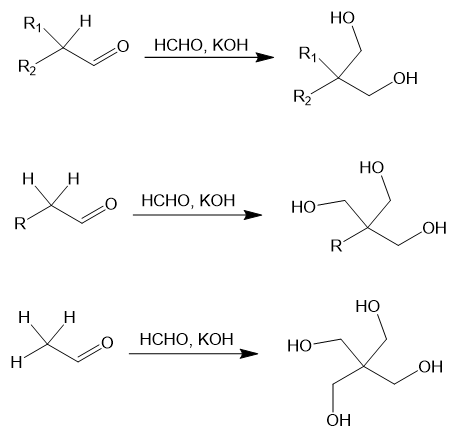
In the Tollens condensation an aldehyde or ketone with at least one α-hydrogen is reacted with formaldehyde and strong base to synthesize diol, triols, or tetrols [1,2]. The reaction sequence involves several mixed adol reactions with formaldehyde followed by a hydride transfer from a Cannizzaro reaction. Ultimately the aldehyde and each of its α-hydrogens are transformed into CH2OH groups. For instance, consider an aldehyde with 1, 2, or 3 α-hydrogens:
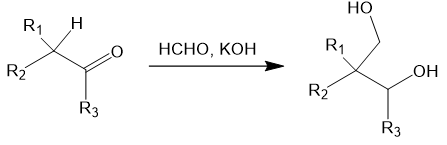
The reaction proceeds similarly if a ketone substrate is used, though the final Cannizzaro reaction now gives a secondary alcohol. For instance:
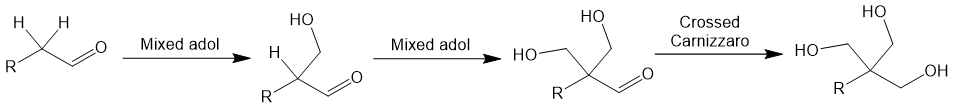
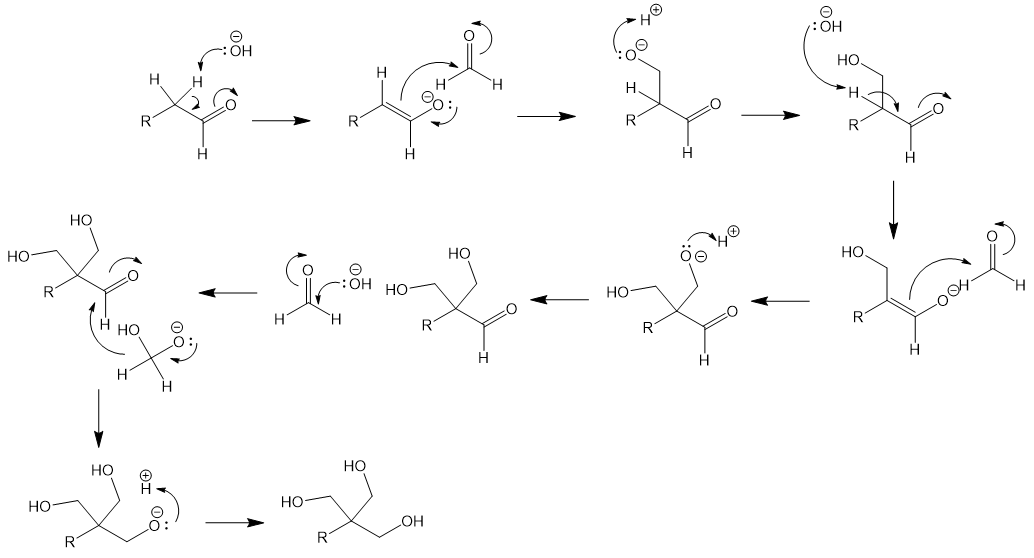
Mechanism of the Tollens condensation
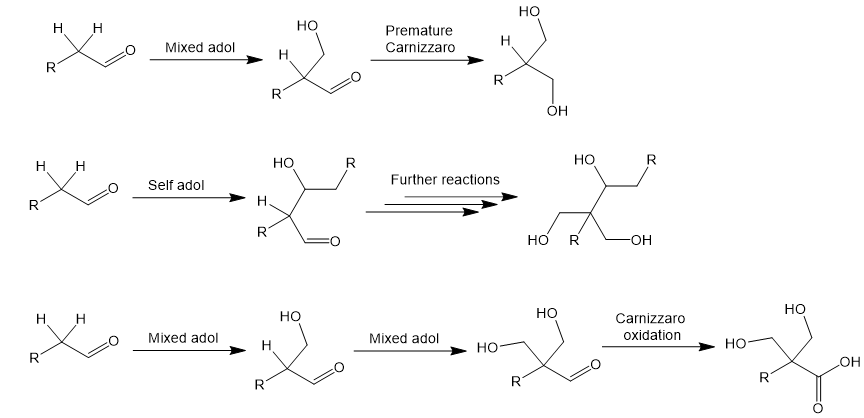
The Tollens reaction is complex, proceeding through multiple intermediates. Here is a stepwise representation of the process using an aldehyde with two α-hydrogens. First, two mixed aldol reactions with formaldehyde replace each α-hydrogen with CH2OH groups. The aldehyde then is reduced to an alcohol by crossed Cannizzaro reaction: a hydride equivalent is transferred from the disproportionation of formaldehyde.
Now in greater detail, I provide an arrow-pushing mechanism for the entire process below. Abstraction of an α-proton by hydroxide generates an enolate. The enolates attacks formaldehyde in a crossed aldol reaction. Protonation of the new alcohol is followed by abstraction of the next α-proton which generates another enolate. Another crossed aldol with formaldehyde occurs. Now there is no α-hydrogens left. A formaldehyde is hydrated by attack of hydroxide. Disproportionation of formaldehyde occurs: a hydride equivalent is transferred to the substrate’s aldehyde, reducing it. Protonation of the alkoxide generates the triol product.
Tollens condensation side products
The desired product from the Tollens condensation requires a very specific sequence of events. Other pathways are possible, giving side products. For this reason, the reaction often gives only low to moderate yields. For instance, suppose the crossed Cannizzaro reaction (reducing the aldehyde to an alcohol) occurs before one or both mixed aldol reactions have taken place. The alcohol product would not be able to react further since an enolate can no longer be generated. Alternatively, the enolate could attack another substrate aldehyde instead of a formaldehyde. Lastly, the aldehyde substrate could participate in a Cannizzaro reaction as the hydride donor, resulting in carboxylic acid side products. Using excess formaldehyde suppresses many of these competing pathways, but some level of them may still occur. In the figure below I give a few examples of side products. Of course, many other combinations of these reactions are possible.
Tollens condensation reaction conditions
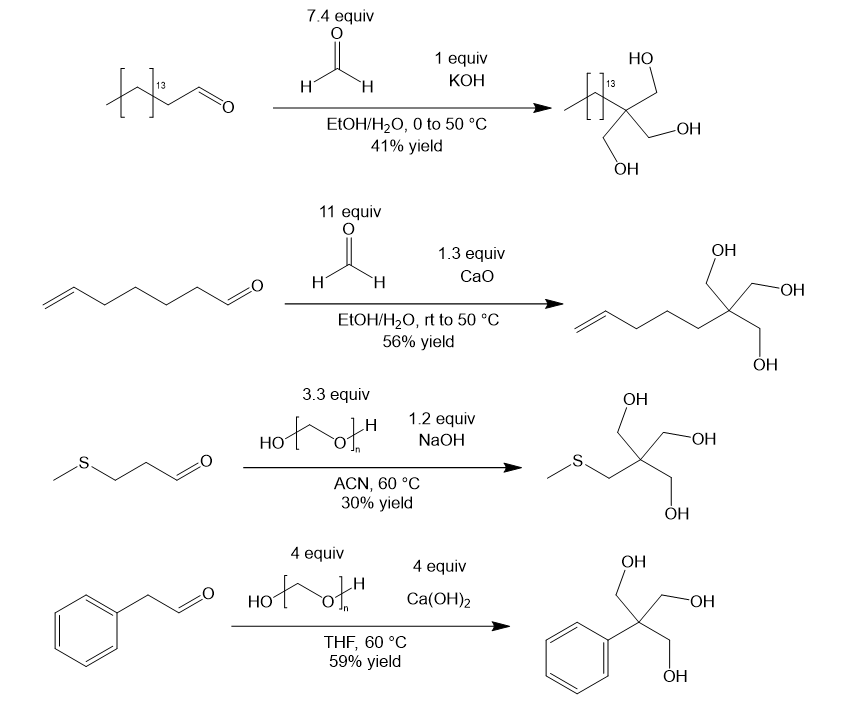
The Tollens reaction uses excess formaldehyde or paraformaldehyde and a strong base (NaOH, KOH, Ca(OH)2, or CaO). Various solvent systems have been reported, but protic solvents like ethanol or water are most common. In the case of paraformaldehyde, aprotic polar solvents like acetonitrile or THF are sometimes used. Here are some representative examples from the literature [3,4,5,6]:
Applications of the Tollens condensation
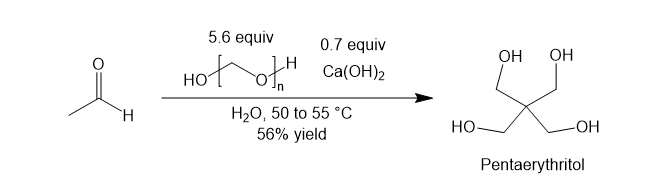
To conclude this article, I give two practical examples of this name reaction to show its usefulness. Most importantly, it is used to produce pentaerythritol, a key building block in the chemical industry. The Tollens reaction of acetaldehyde gives pentaerythritol [7]. This substrate is a special case since it is the only aldehyde with three α-hydrogens. Thus, three aldol reactions occur, resulting in a tetrol.
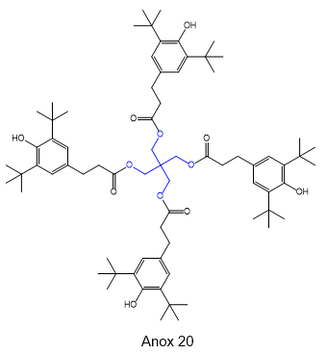
The four hydroxyl groups of pentaerythritol provide a useful scaffold for preparing polyfunctionalized compounds. It finds diverse applications in plastics, adhesives, cosmetics, and paints. For instance, it is needed to make Anox 20, an antioxidant stabilizer used in polypropylene and polyethylene polymers [8]. It is a tetramer of the antioxidant BHT. The increased size greatly reduces its volatility and provides compatibility with the high temperatures required in the production of these plastics. The pentaerythritol core is highlighted in blue:
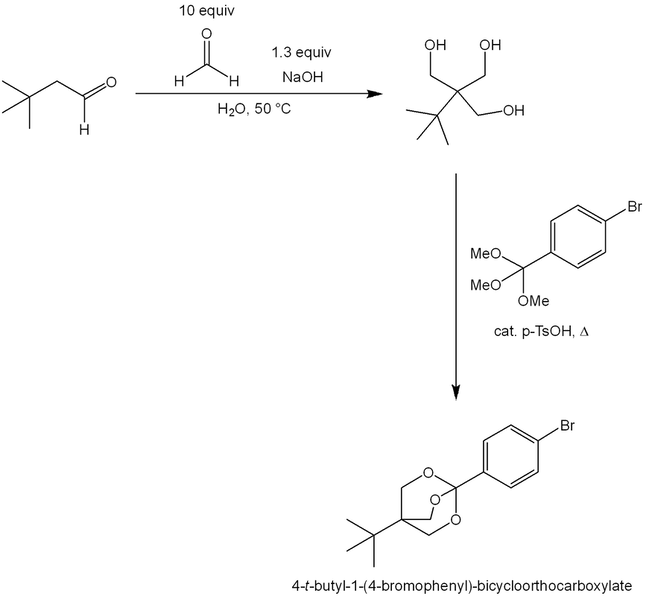
As another example, the Tollens condensation provides access to 4-t-butyl-1-(4-bromophenyl)-bicycloorthocarboxylate, a potent GABA receptor antagonist [9]:
Tollens condensation of 3,3-dimethylbutanal gives a triol intermediate. It is heated with catalytic acid and a trimethyl orthobenzoate derivative, resulting in MeOH liberation and formation of the desired bicyloorthocarboxylate.
 RSS Feed
RSS Feed