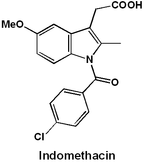
Retrosynthetic analysis of indomethacin
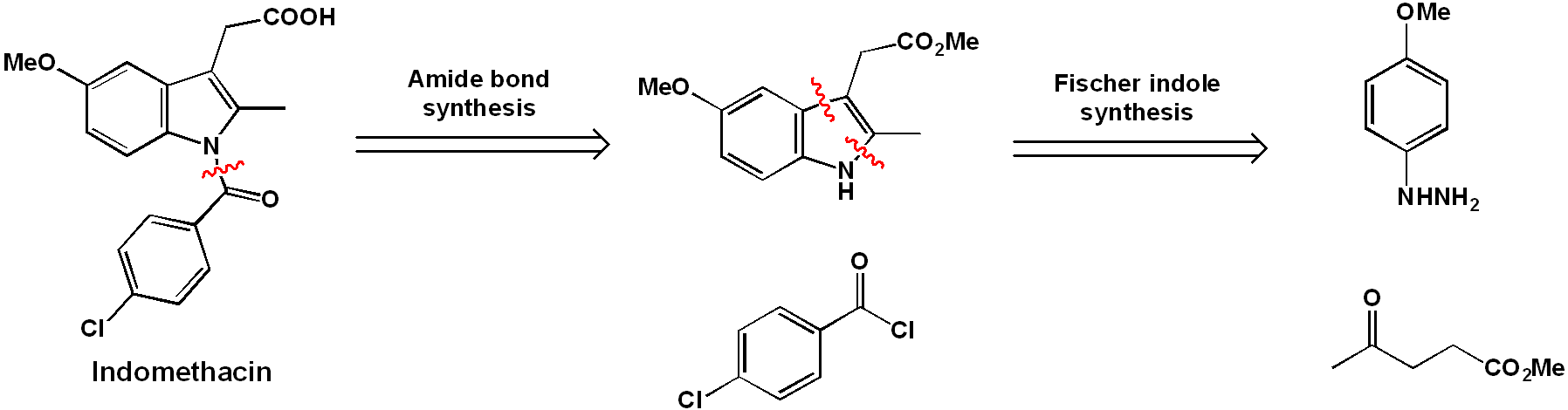
"Traditional" synthesis of indomethacin
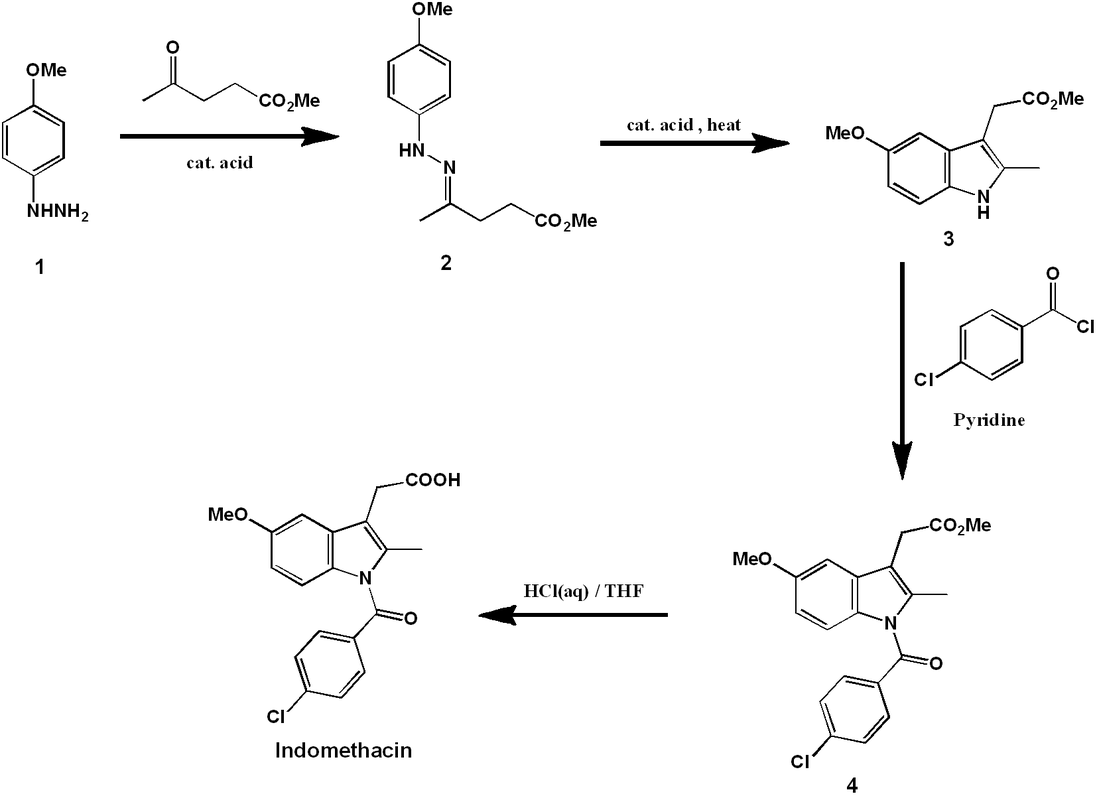
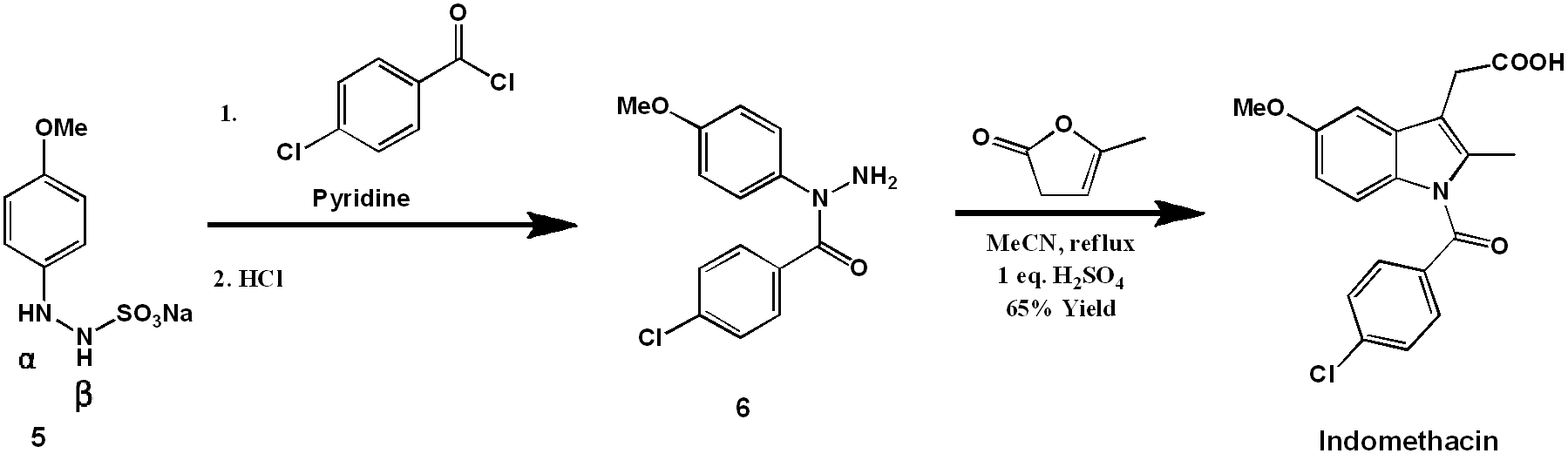
Modern synthesis of indomethacin
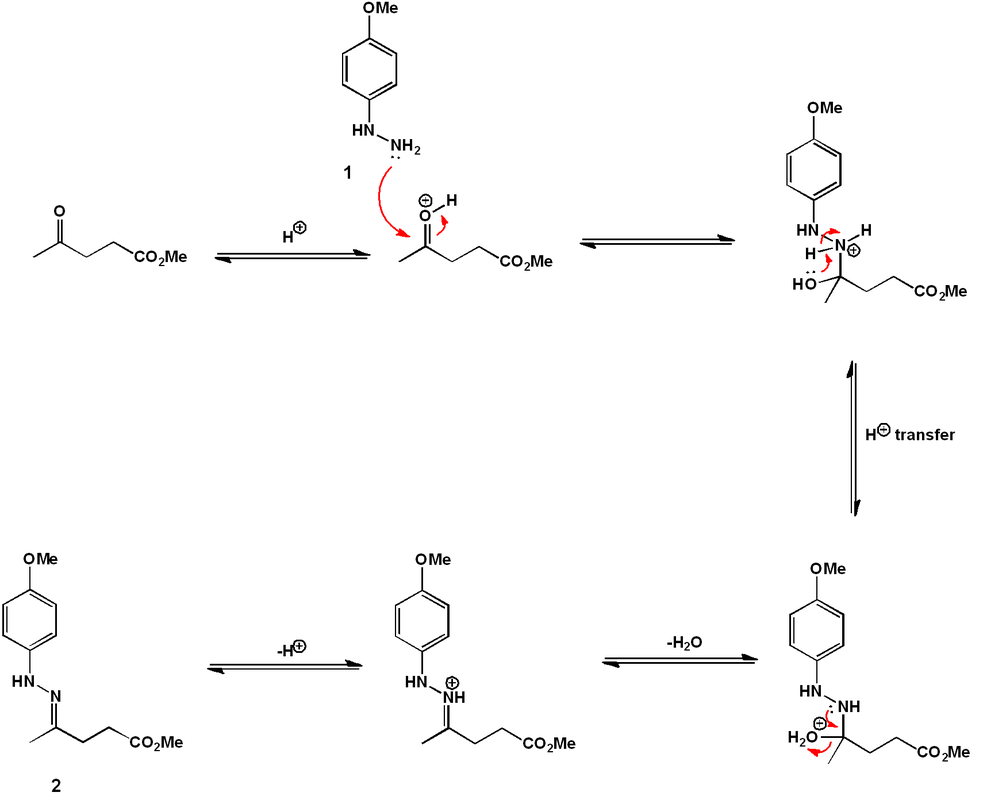
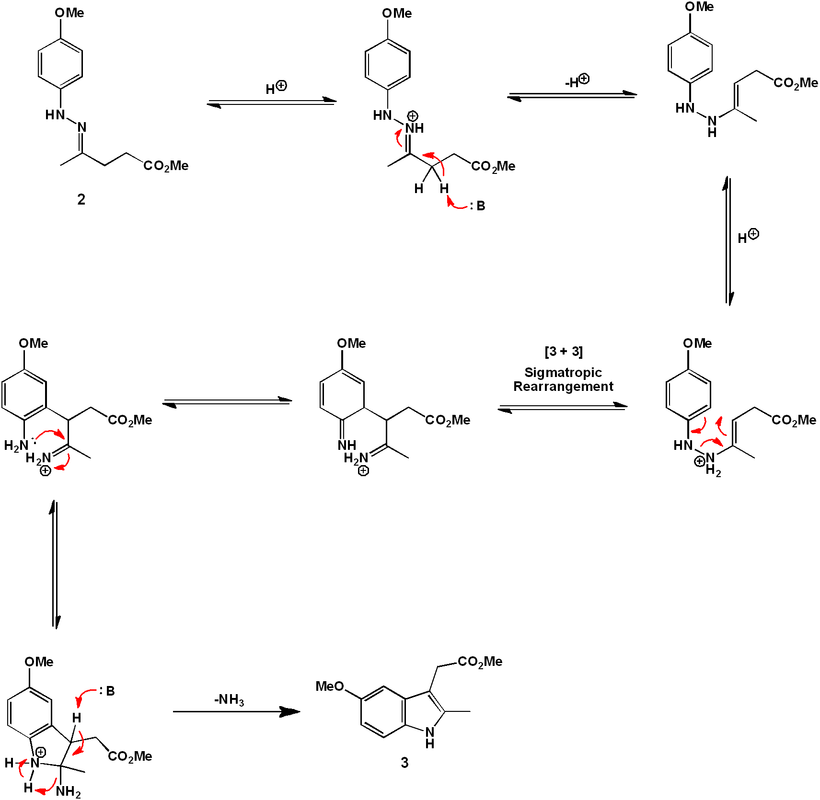
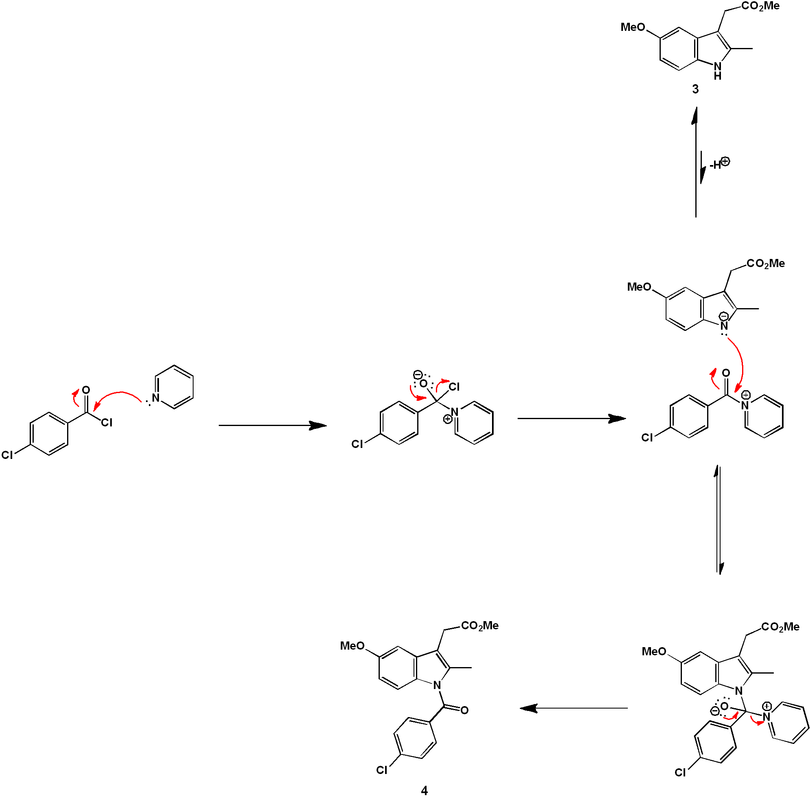
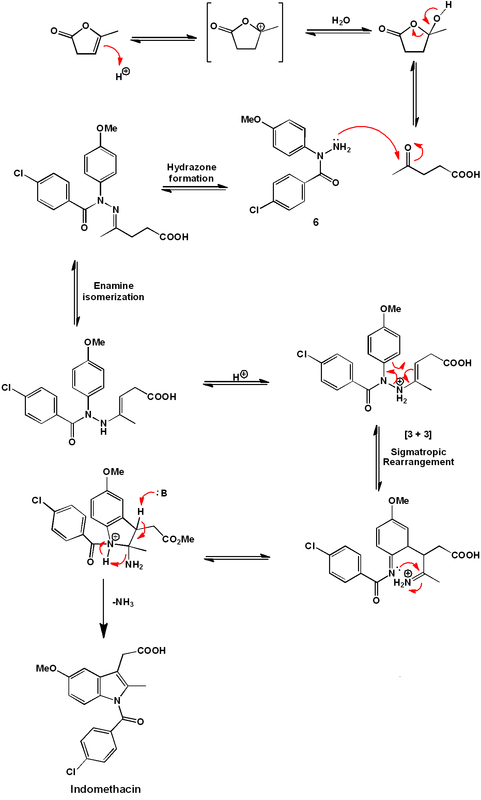
The indole ring is formed by treatment of (6) with α-Angelica lactone under acidic conditions. This dihydropyran functions as a ketone equivalent in the Fischer indole synthesis, as shown mechanistically below. Efficiently, under the conditions developed, the hydrazine formation and the [3 + 3]-sigmatropic rearrangement occur simultaneously under one reaction condition.
References
[2] Karady, S.; Ly, M. G.; Pines, S. H.; Chemerda, J. M.; Sletzinger, M. Synthesis 1973, 50-51.
 RSS Feed
RSS Feed