A system can be said to be living if it is able to transform external matter/energy into an internal process of self-maintenance and production of its own components.
The issue is how highly ordered structural components can be created when only processes which increase the universe's disorder occur spontaneously. Certainly, building complicated biological structures and biomolecules is not by itself spontaneous process. However, such processes can be driven forward by the input of work ("useful" energy), in turn generated through other spontaneous processes. Therefore, the highly ordered nature of life is possible through this coupling. Note that a net gain in entropy is still attained.The conversion of energy into work is not 100% efficient; some energy is always dissipated as unusable heat which increases the entropy of the universe. Living things, therefore, may actually be thought of as heat-dissipating machines, maintaining their order through the production of even larger amounts of disorder. A practical example of this principle follows.
In order for heterotrophs (such as ourselves) to stay alive, we must metabolize food. This provides the required work required to maintain our highly ordered structure. For instance, consider the aerobic respiration of glucose. The reaction below, of course, skips over a very large number of intermediates. However, this is unimportant since G, H and S are all state functions (they depend only on the present state, not the path through which it was achieved).
The question which follows from that example, however, is how sugars like glucose are formed on Earth given the second law. Of course these are formed through photosynthesis which is driven by work from the sun. Fusion reactions occur in the sun, light elements combine to make heavier ones and there is a slight decrease in the total rest mass of the atoms. This releases an incredible amount of energy, some of which can be used by autotrophs on Earth to do work, like synthesizing glucose. Yet much of the energy released by the sun is not available to do work, dissipation of this heat increases the entropy of the universe. When the entropy produced by the sun's fusion is considered, there is no contradiction between life and the second law of thermodynamics. The common error that leads to this mistake is to forget that both the Earth and individual organisms are not isolated systems. Although biological systems have highly ordered structure, they are maintained by work derived from processes that produces even more disorder. The chemistry underlying all biological systems exactly follows the second law of thermodynamics.
References:
[1] Luisi PL. 2006. The emergence of life: From chemical origins to synthetic Biology. New York: Cambridge University Press, 25 p.
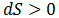
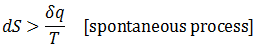
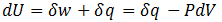
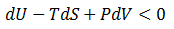
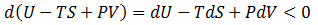
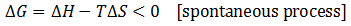
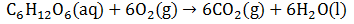
 RSS Feed
RSS Feed