Introduction
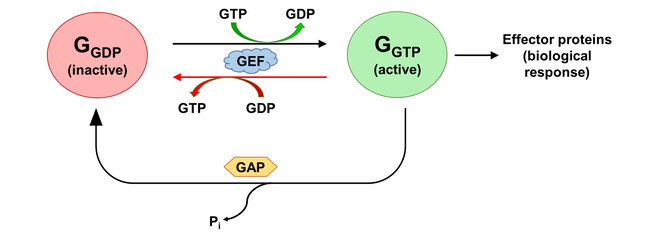
The activation states of a G protein are controlled by the GTPase cycle (Fig. 1). A G protein (GTPase) is considered “active” when bound to guanosine triphosphate (GTP). In this state, the G protein interacts with downstream effector proteins to elicit biological responses. Hydrolysis of GTP to guanosine diphosphate (GDP) deactivates the G protein, which can be re-activated by the exchange of GDP for GTP. The GTPase cycle may be regulated by accessory proteins. Deactivation is facilitated by GTPase activating proteins (GAPs), which couple to GTP-bound G proteins to stimulate their GTPase activity. On the other hand, activation is accelerated by the binding of guanine nucleotide exchange factors (GEFs), which catalyzes GDP/GTP exchange [2]. The mechanism of G protein-GEF interactions and the process of subsequent nucleotide exchange is of special interest to the field of GPCR signalling, where GPCRs fulfill the role of GEF and GTP hydrolysis occur through intrinsic GTPase activity of the heterotrimeric G proteins.
In addition to experimental and computational studies providing extensive structural and biochemical characterization [2-7], mathematical models have been developed for a number of G protein signaling pathways under various conditions [8-14]. Generally, the extent of activation is indicated by either an increase in concentration of an active G protein form (G-GTP in the case of small G proteins, Gα-GTP or Gβγ in the case of heterotrimeric G proteins), or a rise in downstream biological effect such as actin polymerization.
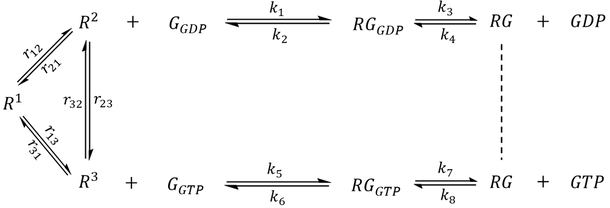
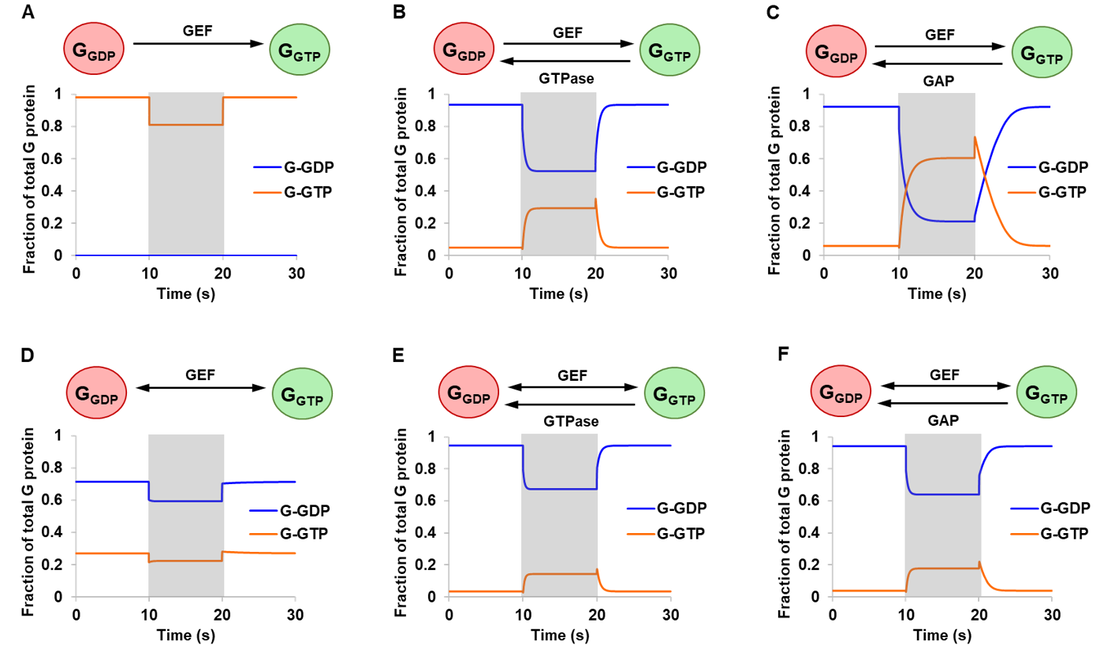
With few exceptions [12-13], GEF-catalyzed nucleotide exchange was considered irreversible. However, it was unclear whether the assumption holds under all circumstances. In their 2016 article, Stanley and Thomas investigated the effect of a reversible GEF mechanism on the behaviour of a small G protein regulatory system using mathematical modelling (Fig. 2) [13]. Their scheme contained a single GEF and a single G protein with either no GTPase activity, intrinsic GTPase activity, or GAP-mediated GTPase activity. The pathway is composed of reversible steps in which an irreversible GEF mechanism could be modelled by disallowing the dissociation of GTP from the GEF-G-GTP complex.
To remain physiologically relevant, kinetic parameters measured for the Ran:RCC1:RanGAP1 system were employed [15-16]. Ran (RAs-related nuclear protein, also known as GTP-binding nuclear protein) is a small 25 kDa G protein located preferentially in the cell nucleus. Together with regulatory proteins RCC1 (a GEF for Ran) and RanGAP1 (a GAP), it is involved in the transport of proteins and mRNAs across the nuclear membrane, as well as DNA synthesis and cell cycle progression [17]. Here, I replicate the simulations performed by Stanley and Thomas, and present an extension to their model. A hypothetical GPCR-G protein signaling pathway was constructed with consideration to functionally significant protein dynamics. The model was progressively augmented to include a multi-state GEF, a GPCR-activating ligand, and a multi-state G protein.
Mathematical models
Initial model
Extension 1: a multi-state GEF
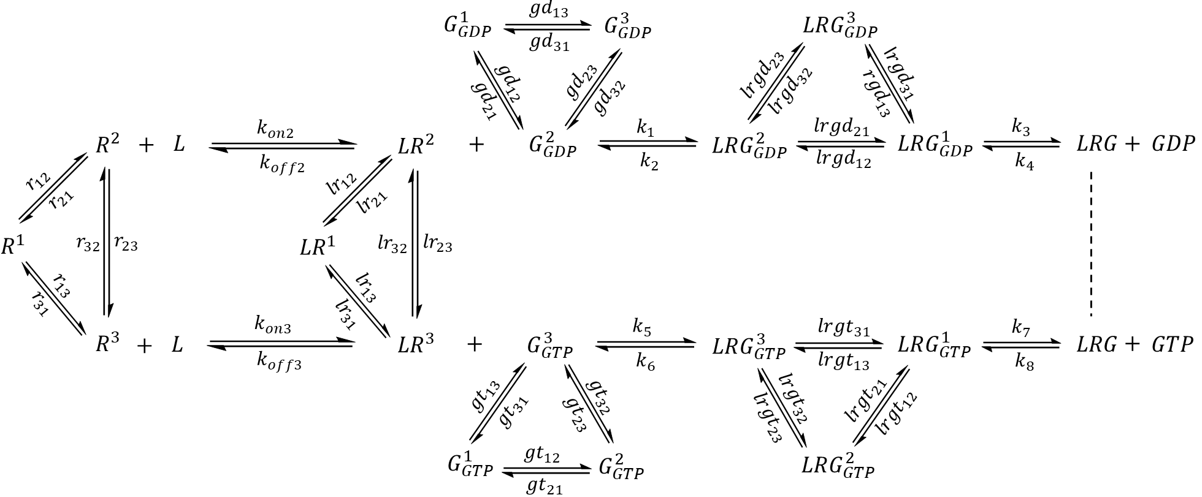
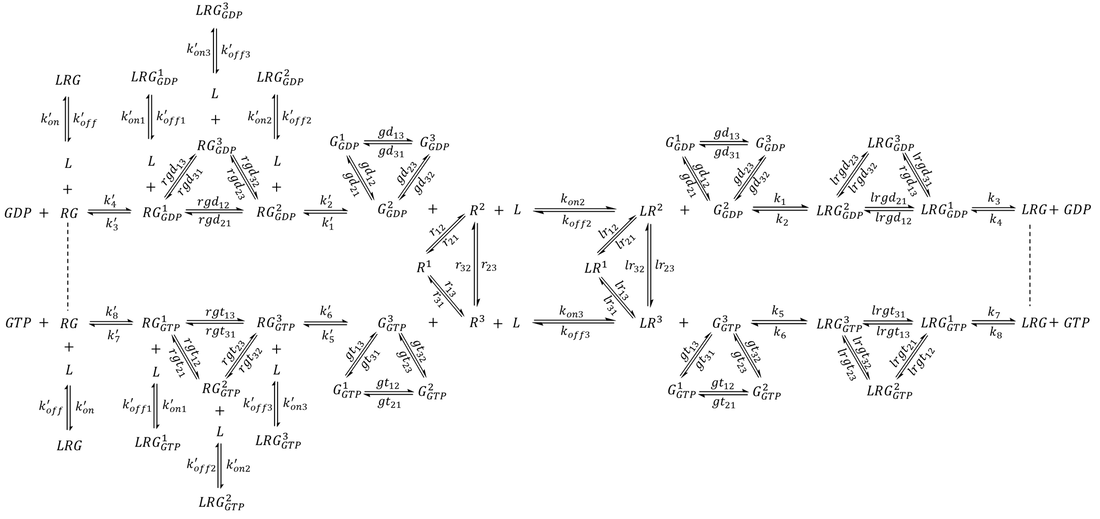
The first step of model extension replaced the generic GEF with a GPCR (R) that adopts three distinct conformational states- an inactive state R1 and two active-like states R2 and R3 capable of binding G protein (Scheme 1). Whereas R2 preferentially couples to G-GDP, the reversibility of GEF is preserved by R3, which preferentially couples to G-GTP. Since each state undergoes exchange with the other two, a receptor may sample all three conformations even in the absence of interacting partners.
The following set of ODEs were derived for Scheme 1 along with the conservation of mass of the receptor and the G protein:
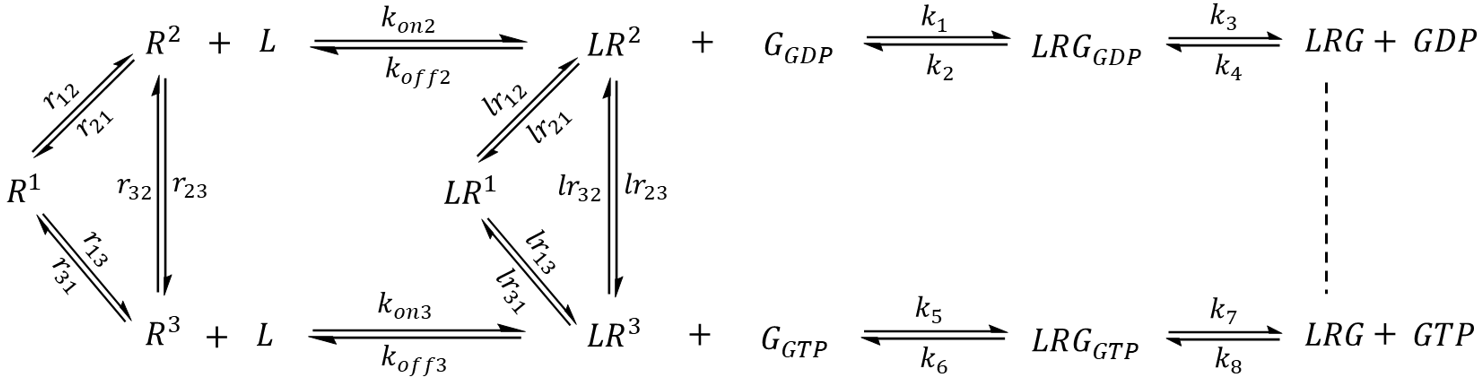
Extension 2: introducing an agonist
The following ODEs were obtained for Scheme 2 along with the conservation of mass of the receptor, the G protein, and the ligand:
Extension 3: a multi-state G protein
An agonist-bound receptor may accelerate nucleotide exchange in the G protein via several processes. The receptor may facilitate nucleotide release by promoting LRG1-GDP, which has a reduced affinity to nucleotides. The binding of activated receptor stabilizes LRG, which assists in the complete dissociation of GDP and the diffusion of GTP to the nucleotide binding site. The subsequent binding of GTP (and to some extent re-association of GDP) significantly reduces receptor-G protein affinity, which helps to produce free active G proteins and regenerate receptors for another round of activation. While the latter two processes have both been observed experimentally [7,23], the conformational landscape of receptor-bound, multi-state G proteins have not yet been studied. To explore this model, the following list of ODEs were simulated:
Results and Discussion
GEF may serve versatile functions in a GTPase cycle
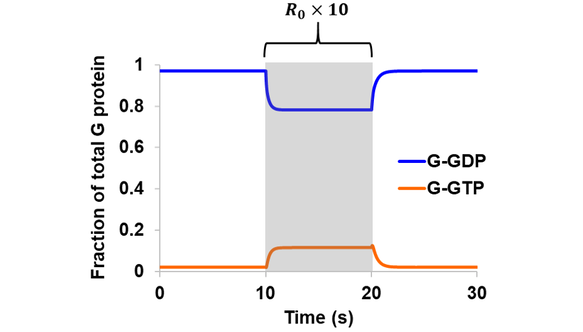
In the absence of GTPase activity, the reversible and the irreversible GEF mechanism showed qualitatively different system behaviour especially with respect to steady state concentrations (Fig. 3A and 3D). The addition of GEF triggered a drop in G-GTP, which can be attributed to its sequestration into GEF-G protein complexes. From these, Stanley and Thomas argued that 1) GEF can be stimulating as well as inhibitory and 2) the assumption of an irreversible model may lead to incorrect conclusions or predictions of system behaviour [13].
System behaviour is a function of changing conformational landscapes
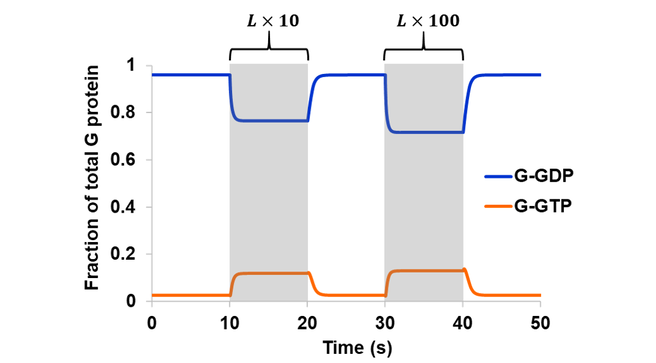
In the second part of model expansion, an external ligand was introduced. In literature, GPCR activation is often described in terms of structural changes in the receptor upon agonist binding, leading to an “active” conformation capable of coupling to G proteins [24]. From an ensemble perspective, however, a receptor samples all available conformations even in its apo form [20]. Perturbations in the environment leads to an altered energy landscape and a redistribution of states. Here, receptor activation is accomplished through an increase in the active-like conformations (LR2 and to some extent LR3) upon ligand binding, which led to an increased level of active G protein (Fig. 5). The degree of system response is a function of ligand concentration. Elevating the receptor-ligand ratio from sub-stoichiometric (10:1) to 1:1 resulted in a 4.5-fold increase in G-GTP. Another 10-fold increase in ligand (1:10 receptor-ligand ratio), approaching saturating concentration, only gave rise to an additional 1.1-fold increase in G-GTP.
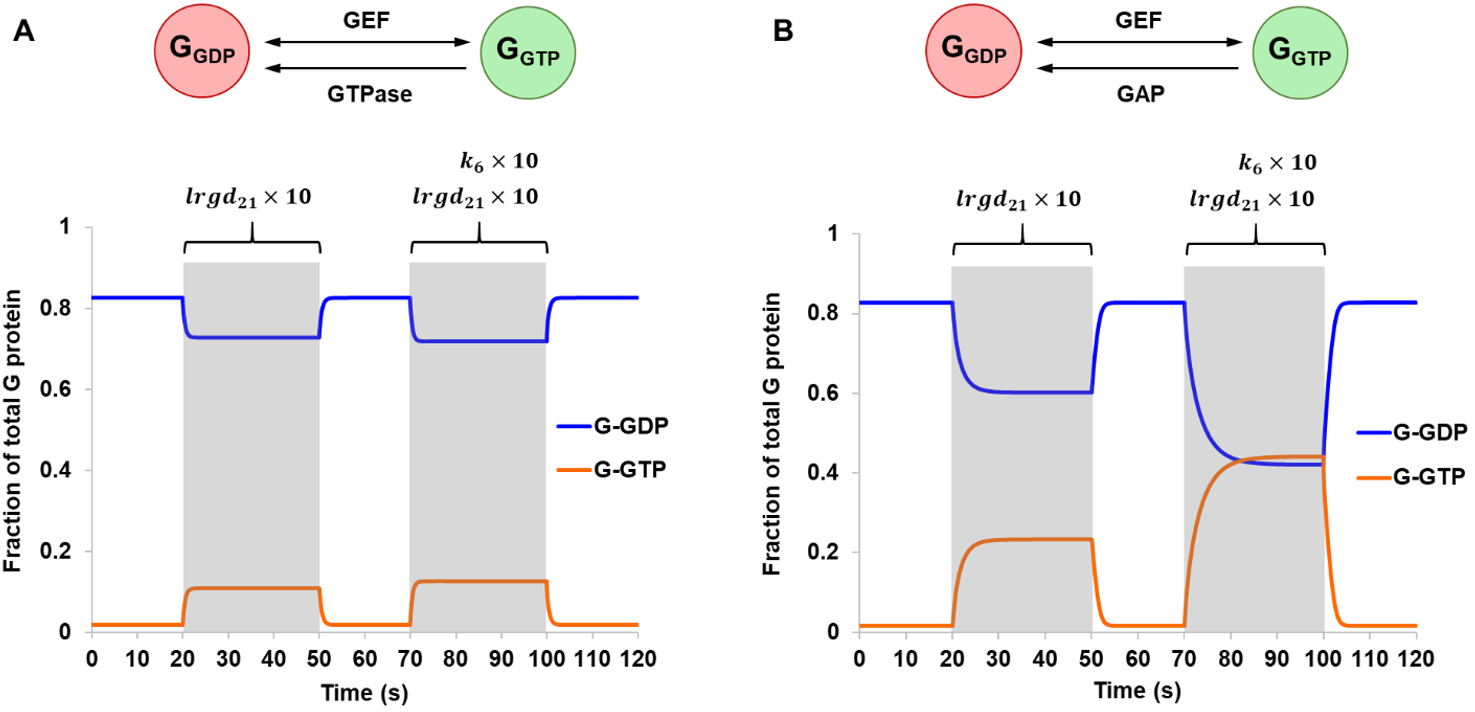
Lastly, a multi-state G protein was considered. In this model, a G protein may sample three major conformational states whose populations and lifetimes are modulated by binding partners such the nucleotides and the agonist-bound receptor. The simulation explored two different methods that a receptor may utilize to catalyze nucleotide exchange (Fig. 6). A receptor may influence the conformational landscape of a G protein to promote LRG1-GDP, a conformation with weaker affinity to nucleotides. Conceptually, this would facilitate GDP release and was reflected in the model by assigning a larger value to the rate constant lrgd21 (Scheme 3). As expected, a 5.8-fold increase in G-GTP was observed during the stimulation period when GTP hydrolysis occurred through intrinsic GTPase, and a 13.9-fold increase was observed in the case of a GAP-mediated GTPase. The system was further activated by increasing k6, the rate constant for the dissociation of G-GTP from LRG-GTP (Fig. 6). This is another step where the receptor may help to drive nucleotide exchange. Here, the effect of activation was much larger for a GAP-mediated GTPase in comparison to an intrinsic GTPase. These results point to the interdependency of different modes of pathway regulation and how they could be utilized in conjunction to control signal amplification levels.
Conclusion and Future Work
Author’s note: the MATLAB codes used, simulation parameters, and data presented in this article are available upon request. This article was originally written in 2017 and has not been updated to consider more recent literature.
References
[2] Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell 129, 865–877 (2007).
[3] Sprang, S. R. Invited review: Activation of G proteins by GTP and the mechanism of Gα-catalyzed GTP hydrolysis. Biopolymers 105, 449–462 (2016).
[4] Vetter, I. R. & Wittinghofer, A. The Guanine Nucleotide-Binding Switch in Three Dimensions. Science. 294, 1299–1304 (2001).
[5] Dror, R. O. et al. Structural Basis for Nucleotide Exchange in Heterotrimeric G Proteins. Science. 348, 1361–1365 (2015).
[6] Goricanec, D. et al. Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proc. Natl. Acad. Sci. 113, E3629–E3638 (2016).
[7] Gregorio, G. G. et al. Single-molecule analysis of ligand efficacy in β2AR-G-protein activation. Nature 547, 68–73 (2017).
[8] Khatibi, S., Rios, K. I. & Nguyen, L. K. in Methods in molecular biology (ed. Rivero, F.) 3–20 (Springer New York, 2018). doi:10.1007/978-1-4939-8612-5_1
[9] Yi, T.-M., Kitano, H. & Simon, M. I. A quantitative characterization of the yeast heterotrimeric G protein cycle. Proc. Natl. Acad. Sci. 100, 10764–10769 (2003).
[10] Hao, N., Yildirim, N., Wang, Y., Elston, T. C. & Dohlman, H. G. Regulators of G protein signaling and transient activation of signaling: Experimental and computational analysis reveals negative and positive feedback controls on G protein activity. J. Biol. Chem. 278, 46506–46515 (2003).
[11] Adams, J. A., Omann, G. M. & Linderman, J. J. A mathematical model for ligand/receptor/G-protein dynamics and actin polymerization in human neutrophils. J. Theor. Biol. 193, 543–560 (1998).
[12] Bornheimer, S. J., Maurya, M. R., Farquhar, M. G. & Subramaniam, S. Computational modeling reveals how interplay between components of a GTPase-cycle module regulates signal transduction. Proc. Natl. Acad. Sci. U. S. A. 101, 15899–15904 (2004).
[13] Stanley, R. J. & Thomas, G. M. H. Activation of G Proteins by Guanine Nucleotide Exchange Factors Relies on GTPase Activity. PLoS One 11, e0151861 (2016).
[14] Bridge, L. J., Mead, J., Frattini, E., Winfield, I. & Ladds, G. Modelling and simulation of biased agonism dynamics at a G protein-coupled receptor. J. Theor. Biol. 442, 44–65 (2018).
[15] Klebe, C., Wittinghofer, A., Bischoff, F. R. & Ponstingl, H. Interaction of the Nuclear GTP-Binding Protein Ran with Its Regulatory Proteins RCC1 and RanGAP1. Biochemistry 34, 639–647 (1995).
[16] Klebe, C., Prinz, H., Wittinghofer, A. & Goody, R. S. The Kinetic Mechanism of Ran-Nucleotide Exchange Catalyzed by RCC1. Biochemistry 34, 12543–12552 (1995).
[17] Hadjebi, O., Casas-Terradellas, E., Garcia-Gonzalo, F. R. & Rosa, J. L. The RCC1 superfamily: From genes, to function, to disease. Biochim. Biophys. Acta - Mol. Cell Res. 1783, 1467–1479 (2008).
[18] MATLAB. 9.5 (R2018b). Mathworks Inc. Natick, MA (2018).
[19] Lohse, M. J. et al. Kinetics of G-protein-coupled receptor signals in intact cells. Br. J. Pharmacol. 153, 125–132 (2008).
[20] Ye, L., Van Eps, N., Zimmer, M., Ernst, O. P. & Prosser, R. S. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 533, 265–268 (2016).
[21] Teilum, K., Olsen, J. G. & Kragelund, B. B. Functional aspects of protein flexibility. Cell. Mol. Life Sci. 66, 2231–2247 (2009).
[22] Lane, J. R., May, L. T., Parton, R. G., Sexton, P. M. & Christopoulos, A. A kinetic view of GPCR allostery and biased agonism. Nat. Chem. Biol. 13, 929–937 (2017).
[23] Rasmussen, S. G. F. et al. Crystal structure of the β¬2 adrenergic receptor – Gs protein complex. Nature 477, 549–555 (2011).
[24] Manglik, A. & Kruse, A. C. Structural Basis for G Protein-Coupled Receptor Activation. Biochemistry 56, 5628–5634 (2017).
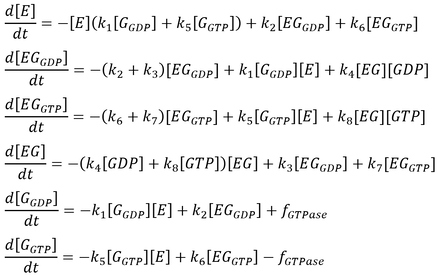
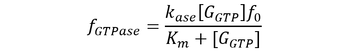
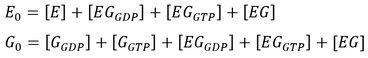
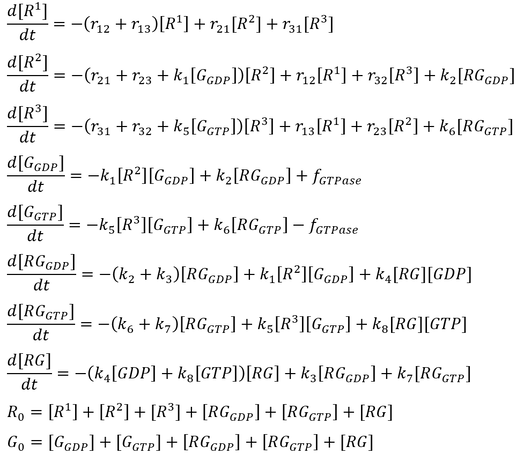


 RSS Feed
RSS Feed