Tremendous efforts have been made to improve NMR signal sensitivity. One approach is to employ hyperpolarization to induce a nuclear spin population difference far greater than that of the thermal equilibrium distribution. Dynamic nuclear polarization (DNP) is a technique where such hyperpolarization is achieved by the transfer of polarization from highly polarized electrons to the nuclei. The technique utilizes the larger population difference in spin states of the electrons in a magnetic field. This is approximately 660 times that of the population difference observed for protons as given by the ratio γe/γn, where γe and γn are the gyromagnetic ratio of the electron and the nucleus, respectively [2]. In practice, an enhancement (ε) in signal-to-noise ratio (S/N) of 40-200 have been observed.
The concept of DNP was first introduced by Overhauser, then experimentally verified by Carver and Slichter for conducting solid metals and subsequently for solutions [3-5]. DNP has gained wide applicability in ssNMR given the larger enhancement which can be obtained and the often requirement for samples to be present at cryogenic temperatures. In a DNP experiment, the sample is doped with a paramagnetic agent which supplies a source of unpaired electrons. Microwave irradiation of the sample at the appropriate electron paramagnetic resonance (EPR) frequencies leads to the transfer of polarization to the bulk nuclear spins and results in DNP enhancement [2]. A brief discussion of the mechanisms of DNP is given in the next section, followed by an overview of instrumentation, sample preparation, and experimental setup. The paper concludes with a case study using the membrane protein bacteriorhodopsin and demonstrates the utility of DNP MAS NMR in studying complex biological systems.
Mechanisms of DNP
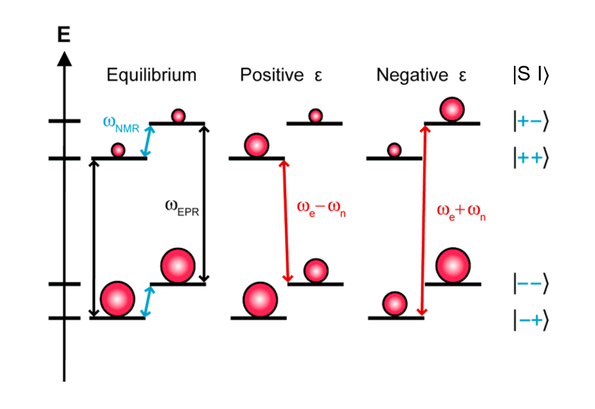
Solid Effect
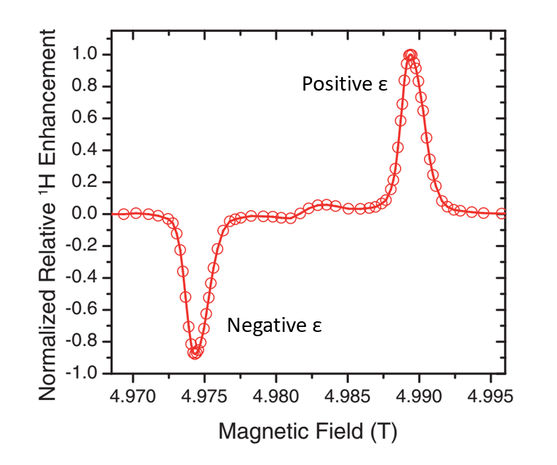
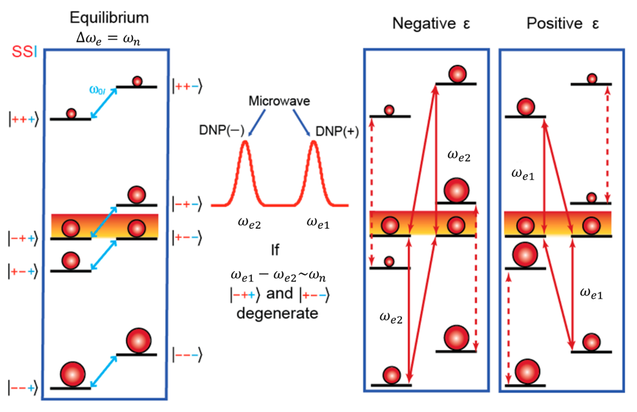
At thermal equilibrium, a very small population difference can be obtained at the NMR transitions. Irradiation is applied at the partially allowed double quantum (DQ, |--⟩ ⟷ |++⟩) or zero quantum (ZQ, |-+⟩ ⟷ |+-⟩) transition frequencies (ωe - ωn and ωe + ωn respectively), leading to saturation and equalized populations of the irradiated states. Consequently, the population difference corresponding to the NMR transitions becomes considerably larger and produces either a positive (double quantum) or a negative (zero quantum) signal enhancement in the NMR spectrum (Fig. 2).
The efficiency of the solid effect is influenced by a number of experimental parameters according to the following expression:
Cross Effect
Spin Diffusion and Polarization Buildup
Typically, an exponential polarization buildup is observed. The polarization buildup time is dependent on the microwave power, the gyromagnetic ratio of the electron and the nuclei, the concentration of the electrons and the nuclei, and relaxation rates. These factors affect the initial electron-nuclear polarization transfer, as well as spin diffusion to the bulk nuclei or nuclei at the site of interest [2]. For a protein sample, polarization buildup times of ~1 s, ~10 s, and ~260 s were observed for protons, carbons, and nitrogens, respectively. These differences could result from variations in the rate and the efficiency of spin diffusion, or from differences in nuclear relaxation times [2].
Instrumentation
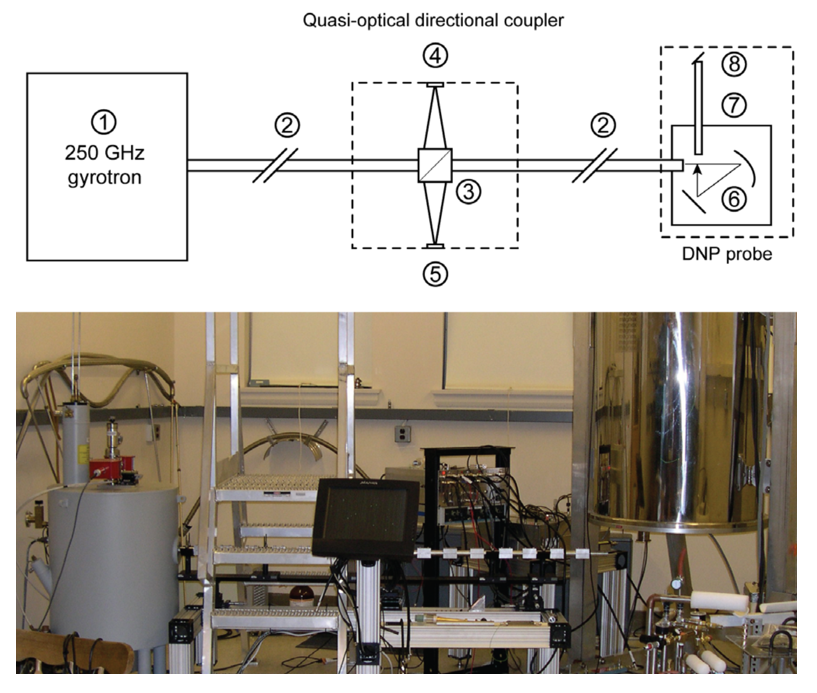
Microwave Source
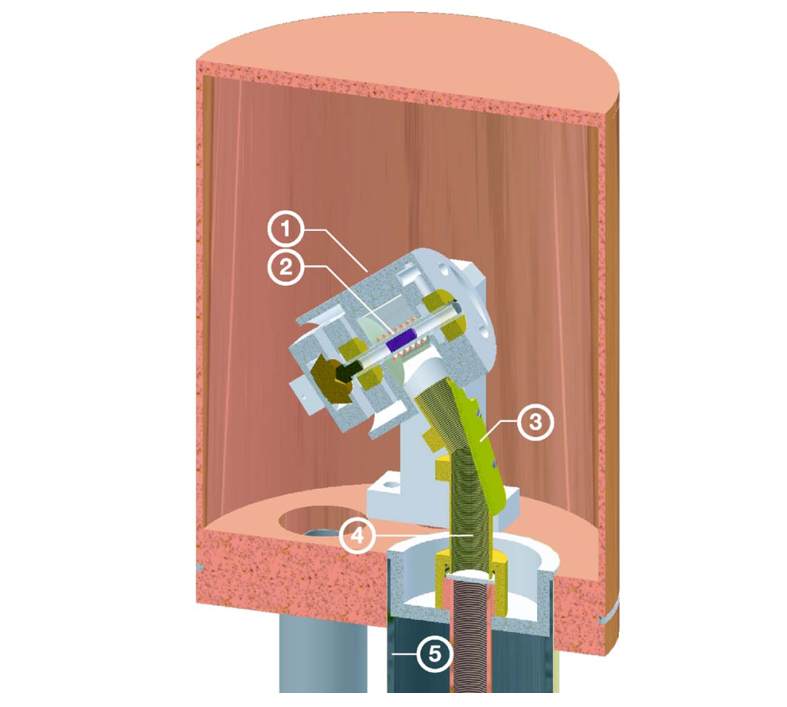
Cryogenic Probes
Sample Preparation
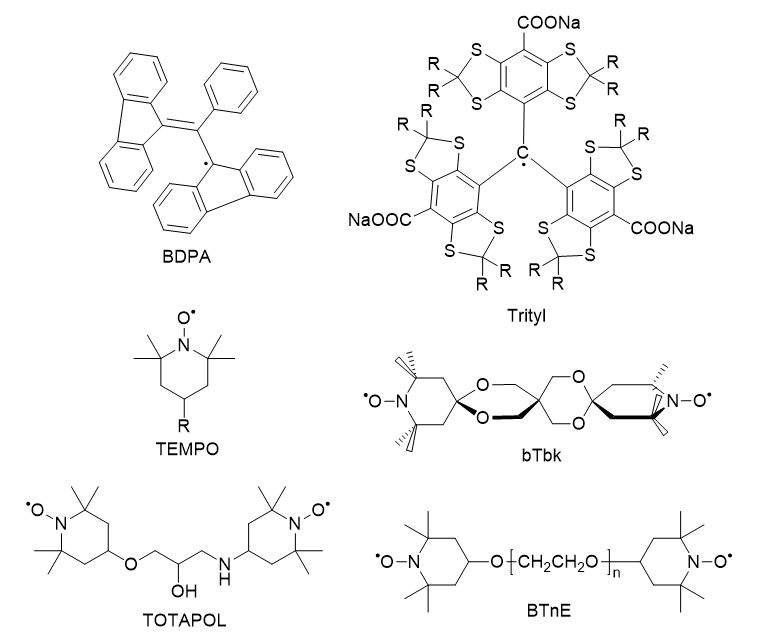
Polarizing Agents
Polarization transfer via CE, the dominant DNP pathway in high fields, requires a specific EPR frequency separation between two electrons in a three-spin system. To this end, biradical paramagnetic agents (molecules containing two unpaired electrons) were developed to improve electron-electron coupling and frequency matching. TEMPO (2,2,6,6-tetramethyl-piperidine-1-oxyls) is a commonly used nitroxide monoradical. By linking two TEMPO molecules through variable linkers, many biradicals were synthesized and tested [16-18]. BT2E (bis-TEMPO-2-ethylene oxide) and TOTAPOL (1-(TEMPO-4-oxy)-3-(TEMPO-4-amino)propan-2-ol) are each composed of two TEMPO moieties separated by a flexible linker (Fig. 6). Electron-electron coupling was significantly improved due to the shortened inter-electron distances (~13 Å), yielding larger DNP enhancements (in comparison to TEMPO) at higher magnetic fields and lower radical concentrations [16].
Through EPR line shape analysis, Hu et al. model the structure of several biradicals and the relative orientation of the g-tensors of the two TEMPO moieties. While the structure of TOTAPOL appears flexible, the g-tensors of its two TEMPO moieties are mostly perpendicular to each other. This yielded a EPR frequency separation which satisfies the CE matching condition. Based on this geometry, the biradical bTbk (bis-TEMPO-bisketal) was synthesized with a rigid bisketal linker in between two TEMPO moieties, restraining their g-tensors in a perpendicular orientation [17]. Despite its lower solubility, a larger DNP enhancement was observed for bTbk than for TOTAPOL under similar conditions.
Radical Concentration
Solvent and Sample Deuteration
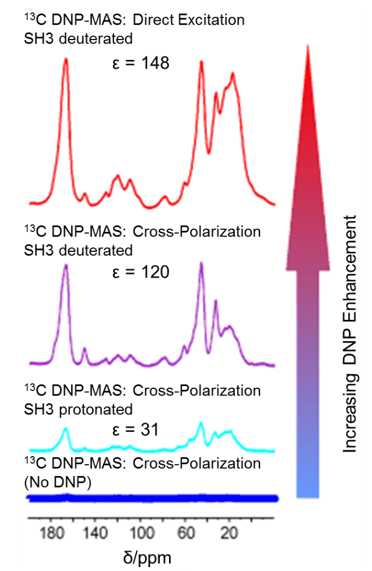
Protein deuteration can also improve DNP enhancement. An increase in factors of roughly 4 and 19 were observed for protons and carbons, respectively, upon deuteration of the protein SH3 in comparison to the fully protonated SH3 [20]. The 13C DNP spectra of deuterated and protonated SH3 is compared in Fig. 7. In addition to the obvious rise in S/N as a result of deuteration, a further increase in signal intensity was observed with direct excitation of 13C compared to indirect detection through polarization transfer. The advances in sample preparation techniques, such as the development of better polarizing agents and sample deuteration, have enabled “high-temperature DNP” experiments (>100 K) while maintaining sufficient DNP enhancement [21]. Higher temperature often results in improved resolution and narrower linewidths, which is especially important for 2D and 3D NMR studies of complex biological systems [2]. The ability to conduct DNP experiments at variable temperatures greatly expanded the utility of DNP-ssNMR.
Conclusion
References
[2] Ü. Akbey, W.T. Franks, A. Linden, M. Orwick-Rydmark, S. Lange, H. Oschkinat, Dynamic nuclear polarization enhanced NMR in the solid-state, Top Curr Chem. 338 (2013) 181–228. https://doi.org/10.1007/128_2013_436.
[3] A.W. Overhauser, Polarization of nuclei in metals, Phys. Rev. 92 (1953) 212–213. https://doi.org/https://doi.org/10.1103/PhysRev.92.411.
[4] T.R. Carver, C.P. Slichter, Experimental verification of the Overhauser nuclear polarization effect, Phys. Rev. 102 (1956) 975–980. https://doi.org/https://doi.org/10.1103/PhysRev.102.975.
[5] L.H. Bennett, H.C. Torrey, High negative nuclear polarizations in a liquid, Phys. Rev. 108 (1957) 499–500. https://doi.org/https://doi.org/10.1103/PhysRev.108.499.
[6] C.D. Jeffries, Polarization of nuclei by resonance saturation in paramagnetic crystals, Phys. Rev. 106 (1957) 164–165. https://doi.org/10.1103/physrev.106.164.
[7] B. Corzilius, A.A. Smith, R.G. Griffin, Solid effect in magic angle spinning dynamic nuclear polarization., J. Chem. Phys. 137 (2012) 054201. https://doi.org/10.1063/1.4738761.
[8] Y. Hovav, A. Feintuch, S. Vega, Dynamic nuclear polarization assisted spin diffusion for the solid effect case, J. Chem. Phys. 134 (2011). https://doi.org/10.1063/1.3526486.
[9] V.S. Bajaj, M.K. Hornstein, K.E. Kreischer, J.R. Sirigiri, P.P. Woskov, M.L. Mak-Jurkauskas, J. Herzfeld, R.J. Temkin, R.G. Griffin, 250 GHz CW gyrotron oscillator for dynamic nuclear polarization in biological solid state NMR, J. Magn. Reson. 189 (2007) 251–279. https://doi.org/10.1016/j.jmr.2007.09.013.
[10] L.R.R. Becerra, G.J.J. Gerfen, B.F.F. Bellew, J.A.A. Bryant, D.A.A. Hall, S.J.J. Inati, R.T.T. Weber, S. Un, T.F.F. Prisner, A.E.E. McDermott, K.W.W. Fishbein, K.E.E. Kreischer, R.J.J. Temkin, D.J.J. Singel, R.G.G. Griffin, A Spectrometer for Dynamic Nuclear Polarization and Electron Paramagnetic Resonance at High Frequencies, J. Magn. Reson. Ser. A. 117 (1995) 28–40. https://doi.org/10.1006/jmra.1995.9975.
[11] C.D. Joye, R.G. Griffin, M.K. Hornstein, K.-N. Hu, K.E. Kreischer, M. Rosay, M.A. Shapiro, J.R. Sirigiri, R.J. Temkin, P.P. Woskov, Operational characteristics of a 14-W 140-GHz gyrotron for dynamic nuclear polarization, IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 34 (2006) 518–523. https://doi.org/10.1109/TPS.2006.875776.
[12] A.B. Barnes, G. De Paëpe, P.C.A. Van Der Wel, K.N. Hu, C.G. Joo, V.S. Bajaj, M.L. Mak-Jurkauskas, J.R. Sirigiri, J. Herzfeld, R.J. Temkin, R.G. Griffin, High-field dynamic nuclear polarization for solid and solution biological NMR, Appl. Magn. Reson. 34 (2008) 237–263. https://doi.org/10.1007/s00723-008-0129-1.
[13] J.H. Ardenkjaer-Larsen, I. Laursen, I. Leunbach, G. Ehnholm, L.-G. Wistrand, J.S. Petersson, K. Golman, EPR and DNP properties of certain novel single electron contrast agents intened for oximetric imaging, J. Magn. Reson. 133 (1998) 1–12. https://doi.org/10.1006/jmre.1998.1438.
[14] C.F. Koelsch, Syntheses with triarylvinylmagnesium bromides. alpha,gamma-bisdiphenylene-beta-phenylallyl, a stable free radical, J. Am. Chem. Soc. 79 (1957) 4439–4441. https://doi.org/10.1021/ja01573a053.
[15] O. Haze, B. Corzilius, A.A. Smith, R.G. Griffin, T.M. Swager, Water-soluble narrow-line radicals for dynamic nuclear polarization, J. Am. Chem. Soc. 134 (2012) 14287–14290. https://doi.org/10.1021/ja304918g.
[16] K.N. Hu, C. Song, H.H. Yu, T.M. Swager, R.G. Griffin, High-frequency dynamic nuclear polarization using biradicals: A multifrequency EPR lineshape analysis, J. Chem. Phys. 128 (2008). https://doi.org/10.1063/1.2816783.
[17] Y. Matsuki, T. Maly, O. Ouari, H. Karoui, F. Le Moigne, E. Rizzato, S. Lyubenova, J. Herzfeld, T. Prisner, P. Tordo, R.G. Griffin, Dynamic nuclear polarization with a rigid biradical, Angew. Chemie - Int. Ed. 48 (2009) 4996–5000. https://doi.org/10.1002/anie.200805940.
[18] C. Sauvee, M. Rosay, G. Casano, F. Aussenac, R.T. Weber, O. Ouari, P. Tordo, Highly efficient, water-soluble polarizing agents for dynamic nuclear polarization at high frequency, Angew. Chemie - Int. Ed. 52 (2013) 10858–10861. https://doi.org/10.1002/anie.201304657.
[19] S. Lange, A.H. Linden, Ü. Akbey, W. Trent Franks, N.M. Loening, B.J. Van Rossum, H. Oschkinat, The effect of biradical concentration on the performance of DNP-MAS-NMR, J. Magn. Reson. 216 (2012) 209–212. https://doi.org/10.1016/j.jmr.2012.01.002.
[20] Ü. Akbey, W.T. Franks, A. Linden, S. Lange, R.G. Griffin, B.J. Van Rossum, H. Oschkinat, Dynamic nuclear polarization of deuterated proteins, Angew. Chemie - Int. Ed. 49 (2010) 7803–7806. https://doi.org/10.1002/anie.201002044.
[21] Ü. Akbey, A.H. Linden, H. Oschkinat, High-Temperature Dynamic Nuclear Polarization Enhanced Magic-Angle-Spinning NMR, Appl. Magn. Reson. 43 (2012) 81–90. https://doi.org/10.1007/s00723-012-0357-2.
[22] Matsuki, Y., Idehara, T., Fukazawa, J. & Fujiwara, T. Advanced instrumentation for DNP-enhanced MAS NMR for higher magnetic fields and lower temperatures. J. Magn. Reason. 264, (2017) 107–105.
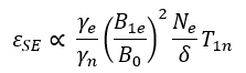
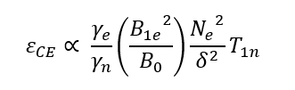
 RSS Feed
RSS Feed