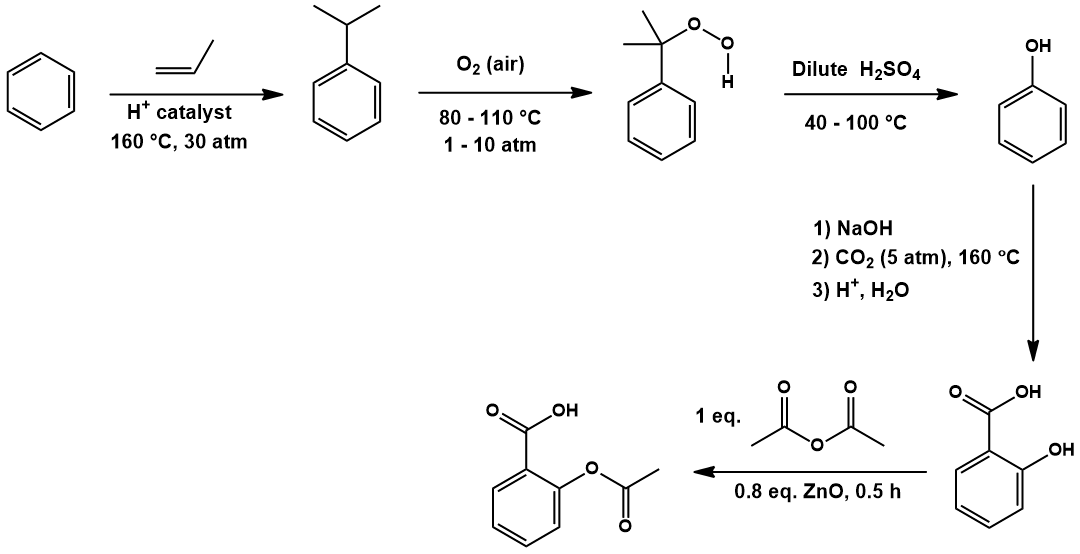
Aspirin (acetylsalicylic acid) is a nonsteroidal anti-inflammantory drug (NSAID) useful for alleviating pain, fever, and inflammation. In addition to being one of the most widely used drugs (44,000 tons consumed globally per year), it is also one of the earliest pharmaceuticals developed, being named “Aspirin” by Bayer in 1899 [1]. In this article, I explore the industrial production of Aspirin, showing how it can be synthesized from benzene in three steps.
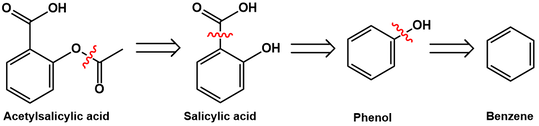
A retrosynthetic analysis is presented below, accessing acetylsalicylic acid from benzene:
Recognizing the acetyl group in Aspirin, this can be prepared by acetylating salicylic acid. Salicylic acid, in turn, is available from ortho-carboxylation of phenol. Lastly, phenol synthesis is possible through hydroxylation of benzene. I will go through these transformations in detail, explaining the reaction conditions and providing arrow-pushing mechanisms for each.
Phenol synthesis from benzene
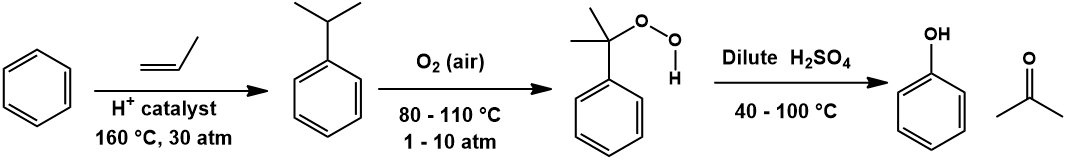
Industrially, phenol is mainly produced through the Cumene process [2]. This transforms benzene and propylene (widely available petrochemicals) into phenol and acetone.
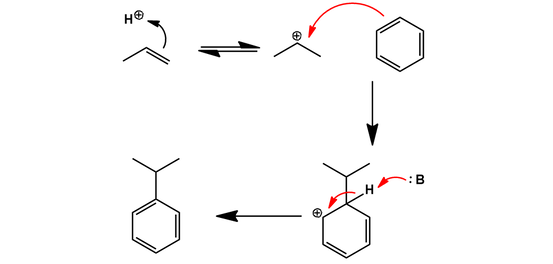
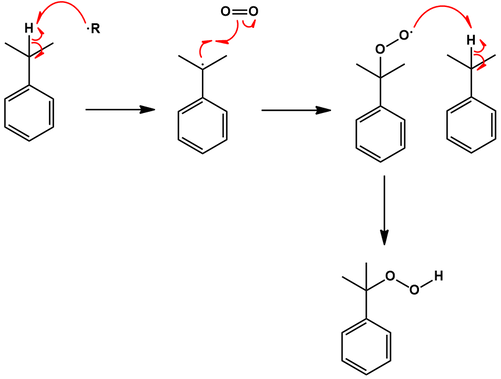
First, benzene is converted to cumene (isopropylbenzene) by Friedel-Crafts alkylation with propylene. Protonation of propylene favors the more stable secondary carbocation, resulting in selective alkylation of benzene with an isopropyl group instead of a linear propyl group:
Next, cumene is oxidized by air to cumene hydroperoxide. Mechanistically, a benzylic radical of cumene is formed which then reacts with molecular oxygen to give the hydroperoxide:
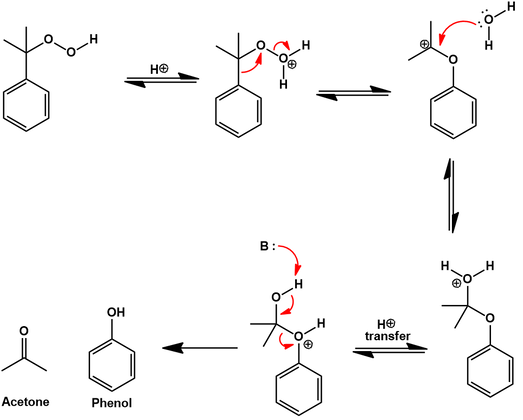
Phenol is obtained by decomposing the cumene hydroperoxide in the presence of dilute aqueous acid. The mechanism for this transformation begins with protonation of the hydroperoxide. This allows expulsion of a water molecular with rearrangement to an isopropyl carbocation ether. Attack of a water molecular at this carbocation center is followed by decomposition to the products acetone and phenol:
Salicylic acid synthesis by the Kolbe-Schmitt reaction
In the next step of industrial Aspirin production, phenol is ortho-carboxylated to salicylic acid. This is accomplished by a Kolbe-Schmitt reaction, using strong base and carbon dioxide gas [3].
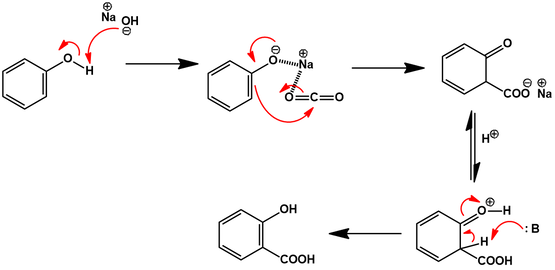
Treatment of phenol with sodium hydroxide generates an electron rich sodium phenolate. This species reacts as an enolate nucleophile, doing a nucleophilic attack on the carbon dioxide. The ortho regiochemistry is favored over para by coordination of the incoming CO2 by the sodium cation. An acidic work-up protonates the carboxylic acid and allows tautomerization to restore aromaticity. An arrow-pushing mechanism is proposed below:
Acetylation of salicylic acid
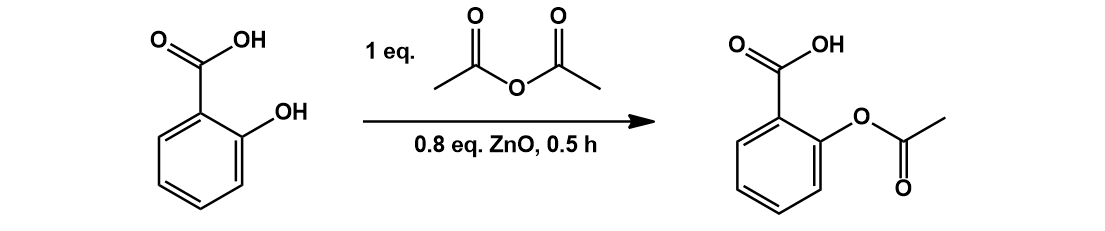
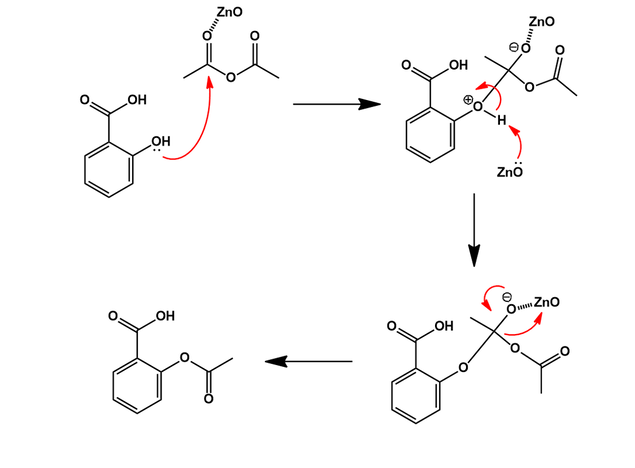
The final step of Aspirin synthesis is acetylation of the salicylic with acetic anhydride or acetyl chloride. Various acid or base catalysts can be used to promote this chemistry. In a patented procedure shown below [4], salicylic acid is reacted with one equivalent of neat acetic anhydride in the presence of zinc oxide:
The reaction is exothermic, spontaneously heating up and proceeding to completion quickly. The zinc oxide catalyzes the acetylation by coordinating the carbonyl oxygen of acetic anhydride, increasing its electrophilicity. Thus, a nucleophilic attack at this carbon center by the phenol of salicylic acid is promoted. Zinc oxide also acts as a base in this reaction, giving zinc acetate as a by-product.
Summary: total synthesis of Aspirin from benzene
First, benzene is alkylated to cumene (isopropylbenzene) by Friedel-Crafts reaction. Oxidation by air gives cumene hydroperoxide, which is then hydrolyzed to phenol by treatment with aqueous acid. Salicylic acid is obtained by othro-carboxylation with carbon dioxide in a Kolbe-Schmitt reaction. Lastly, acetylation with acetic anhydride affords acetylsalicylic acid (Aspirin).
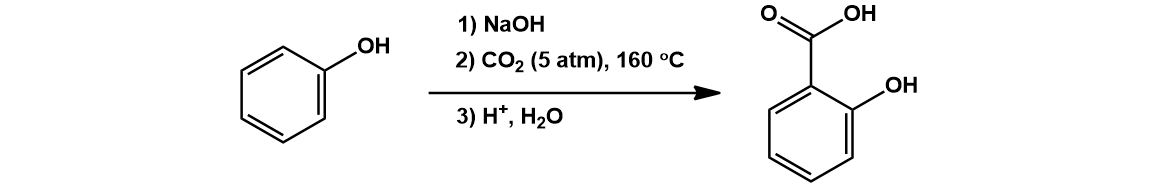
 RSS Feed
RSS Feed