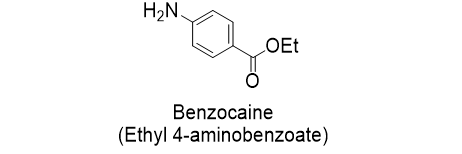
Benzocaine is local anesthetic useful as a topical anesthetic and painkiller. In this article I examine two industrially relevant routes to synthesize this drug from widely available petrochemical building blocks: toluene and p-xylene. Where possible, I provide arrow-pushing mechanisms for the reactions used. Benzocaine belongs to the amino ester class of drugs. It has a simple chemical structure, even having a comprehensible IUPAC name: ethyl 4-aminobenzoate. Installation of the amine and ethyl ester are the key transformations required in the production of this drug.
Synthesis of benzocaine from toluene
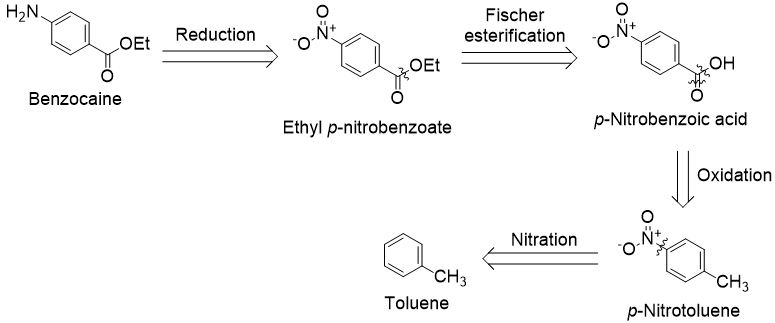
First, I discuss the production of benzocaine from toluene. It is helpful to begin with a retrosynthetic analysis to clarify the logic of the synthetic strategy:
We begin with a functional group interconversion on the aryl amine. It can be obtained from a nitro group by reduction. Earlier in the synthesis, the introduction of a nitro group will be a practical way to functionalize para to toluene’s methyl group. Furthermore, unveiling the amine as the final step avoids complications from its reactivity. Next, we disconnect the ethyl ester. It is available from a Fischer esterification of p-nitrobenzoic acid. In turn, oxidation of p-nitrotoluene’s methyl group gives this derivative. Lastly, the required p-nitrotoluene can be made by nitration of toluene.
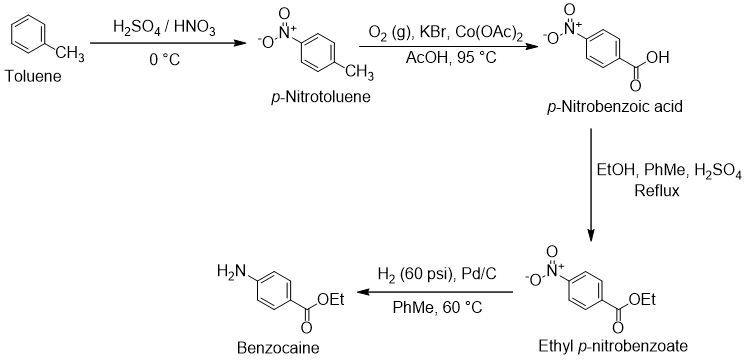
Here is the synthetic route with more detailed reaction conditions:
Here is the synthetic route with more detailed reaction conditions:
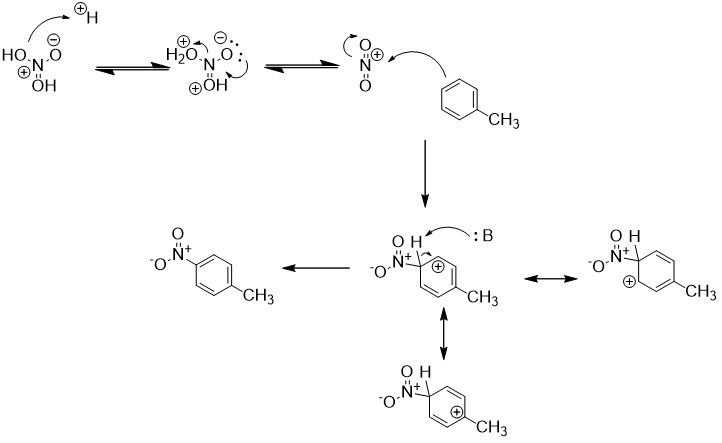
To prepare benzocaine from toluene, the first step is nitration using concentrated sulfuric acid and nitric acid. Though only the para regioisomer p-nitrotoluene is desired for this synthesis, the reaction gives a mixture of ortho and para regioisomers along with a trace of meta [1]. These other nitration products are not a serious problem as they all are valuable building blocks in the chemical industry. They can be separated by fractional distillation and crystallization. Here is an arrow-pushing mechanism for the para-nitration of toluene:
First, protonation of nitric acid and dehydration generates a nitronium ion which is a reactive electrophile. Nucleophilic attack by toluene gives both ortho and para nitrotoluene products. Nitration at these positions is favored over meta due to better resonance stabilization of the arenium ion (the methyl group is electron-donating).
The second step in the synthetic route is the oxidation of p-nitrotoluene to p-nitrobenzoic acid. There are many reactions which would be suitable for this benzylic oxidation. In the condition shown, the reaction is done using oxygen gas and acetic acid as the solvent with catalytic cobalt acetate and potassium bromide [2].
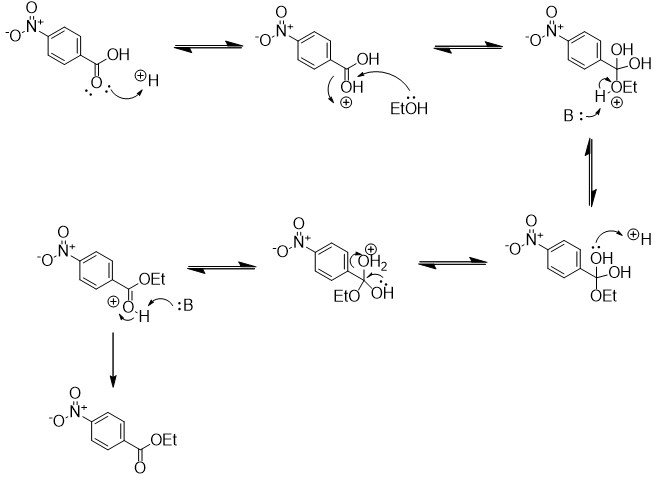
Next, the p-nitrobenzoic acid is esterified with ethanol by Fischer esterification to ethyl p-nitrobenzoate [2]. This reaction is conducted in refluxing ethanol with catalytic sulfuric acid. Toluene is added as a cosolvent to improve solubility. Here is an arrow-pushing mechanism for this Fischer esterification:
The second step in the synthetic route is the oxidation of p-nitrotoluene to p-nitrobenzoic acid. There are many reactions which would be suitable for this benzylic oxidation. In the condition shown, the reaction is done using oxygen gas and acetic acid as the solvent with catalytic cobalt acetate and potassium bromide [2].
Next, the p-nitrobenzoic acid is esterified with ethanol by Fischer esterification to ethyl p-nitrobenzoate [2]. This reaction is conducted in refluxing ethanol with catalytic sulfuric acid. Toluene is added as a cosolvent to improve solubility. Here is an arrow-pushing mechanism for this Fischer esterification:
The mechanism begins with protonation of the carbonyl oxygen of the carboxylic acid. This renders the carbonyl carbon electrophilic. Nucleophilic attack by ethanol is followed by dehydration to give the ethyl ester. The reaction is reversible and using a large excess of ethanol is required to drive the equilibrium toward ester formation.
The final reaction in this route is reduction of the nitro group of ethyl p-nitrobenzoate to an amine, giving the desired benzocaine. This can be accomplished by palladium on carbon-catalyzed hydrogenation [3].
The final reaction in this route is reduction of the nitro group of ethyl p-nitrobenzoate to an amine, giving the desired benzocaine. This can be accomplished by palladium on carbon-catalyzed hydrogenation [3].
Synthesis of benzocaine from p-xylene
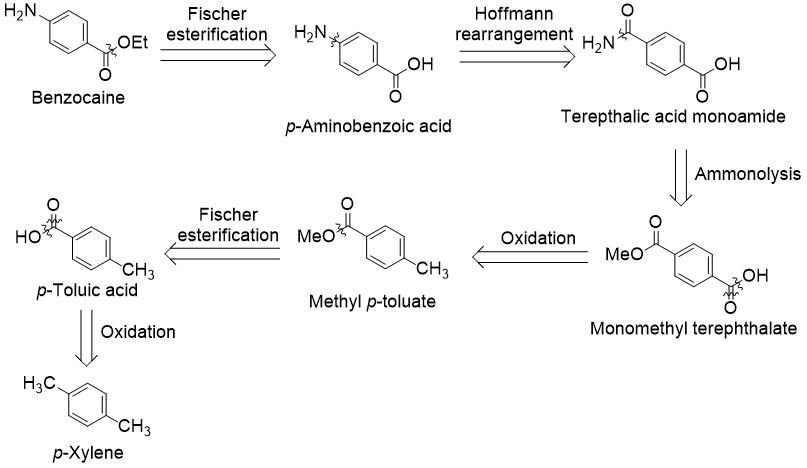
An alternative industrial route to benzocaine begins with p-xylene. As before, I begin with a retrosynthetic analysis exploring this strategy:
This time, we begin the retrosynthesis with the ethyl ester using a Fischer esterification. Next, the amine is disconnected via a Hoffmann rearrangement. The required aryl amide can be produced from monomethyl terephthalate by ammonolysis reaction. Two oxidations and another Fischer esterification leaves us with the starting material p-xylene.
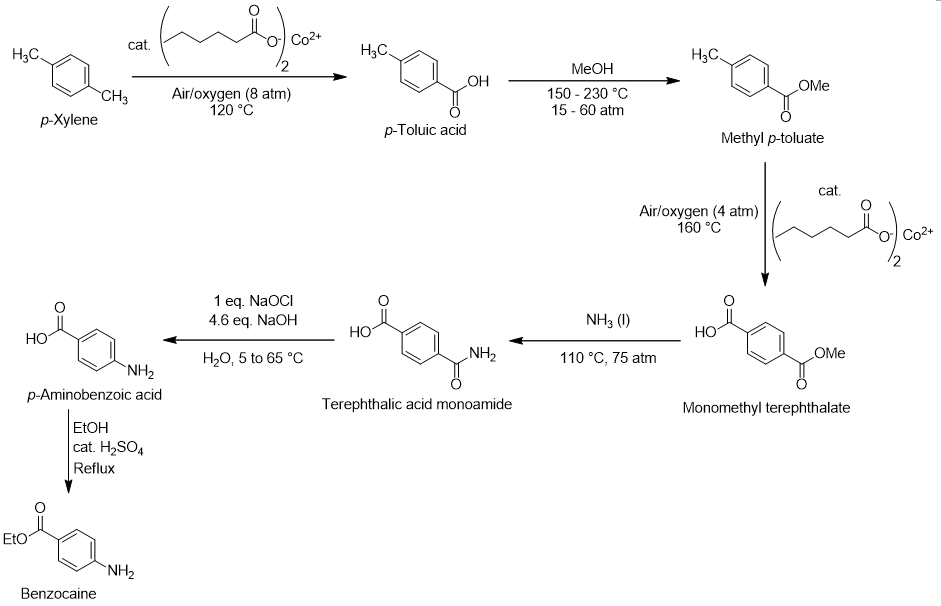
The p-xylene route with detailed reaction conditions follows:
The p-xylene route with detailed reaction conditions follows:
The first three steps of this route, converting p-xylene to monomethyl terephthalate, are known as the Dynamit-Nobel (Witten) process [4]. First, p-xylene is oxidized at one of its benzylic positions to the benzoic acid derivative p-toluic acid. Though there are many conditions this selective oxidation could be accomplished under, the approach shown uses 8 atm of air (oxygen source), 120 °C, and catalytic cobalt fatty acid salt. Next, the p-toluic acid is esterified with methanol to methyl p-toluate. This reaction is done at high temperature (150 – 230 °C) and pressure (15-60 atm), so catalytic strong acid is not required. Mechanistically, this process is equivalent to the Fischer esterification explained above. Another round of air oxidation catalyzed by catalytic cobalt fatty acid salt oxidizes the remaining methyl group, giving monomethyl terephthalate. Ammonolysis with liquid ammonia converts the methyl ester into a primary amide, terephthalic acid monoamide [5].
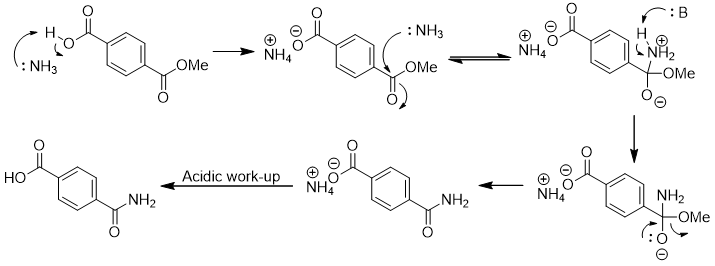
Here is an arrow-pushing mechanism for ammonolysis of the methyl ester:
Here is an arrow-pushing mechanism for ammonolysis of the methyl ester:
Attack of ammonia at the carbonyl carbon of the methyl ester gives a tetrahedral intermediate. Loss of methanol gives the primary amide.
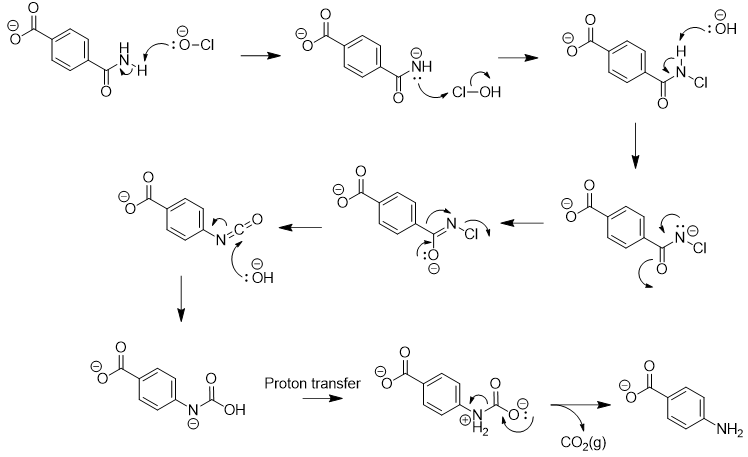
In the next synthetic step, a Hoffmann rearrangement is used to convert the primary amide into an arylamine. The reaction uses stoichiometric sodium hypochlorite (bleach) and excess sodium hydroxide under aqueous conditions [5]. An arrow-pushing diagram for the rearrangement is shown below:
In the next synthetic step, a Hoffmann rearrangement is used to convert the primary amide into an arylamine. The reaction uses stoichiometric sodium hypochlorite (bleach) and excess sodium hydroxide under aqueous conditions [5]. An arrow-pushing diagram for the rearrangement is shown below:
The mechanism begins with deprotonation of the amide and chlorination with hypochlorite. Basic conditions promote rearrangement of the bromoamide to an isocyanate. Hydrolysis and decarboxylation affords the amine.
The final reaction is another Fischer esterification of the p-aminobenzoic acid with ethanol and catalytic sulfuric acid, giving benzocaine. Though the aryl amine is nucleophilic, the acidic conditions and large excess of ethanol supress formation of amide side product.
The final reaction is another Fischer esterification of the p-aminobenzoic acid with ethanol and catalytic sulfuric acid, giving benzocaine. Though the aryl amine is nucleophilic, the acidic conditions and large excess of ethanol supress formation of amide side product.
 RSS Feed
RSS Feed