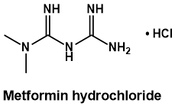
Metformin is a drug used for treating type 2 diabetes, where it works to lower blood sugar levels. It is chemically unusual because of its remarkably high nitrogen:carbon ratio > 1:1. In this article, I explore the organic synthesis of metformin, showing how it can be prepared from simple hydrocarbons. This prospective offers insights into aspects of the chemical industry and how synthetic chemistry can be used to iteratively build structural complexity from the most basic starting materials.
As with all synthetic challenges, we begin with a retrosynthetic analysis.
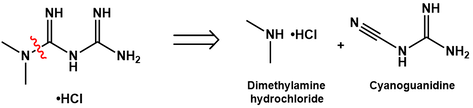
Noting the dimethylamine moiety of metformin, this is a convenient site for retrosynthetic disconnection. This N-C bond can be made by nucleophilic attack of dimethylamine at the electrophilic nitrile carbon of cyanoguanidine. In fact, this is how metformin is industrially prepared. There are various patented procedures which differ in reaction conditions and purification strategy, but almost all involve reacting the hydrochloride salt of dimethylamine with cyanoguanidine [1,2]. An arrow pushing mechanism for this chemistry is proposed below.
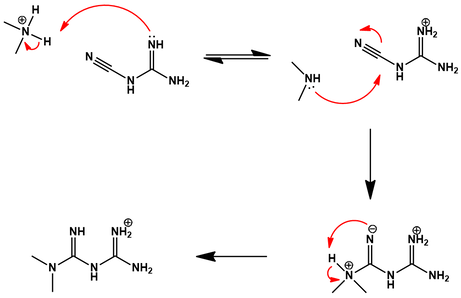
First, the dimethylamine hydrochloride is deprotonated by cyanoguanidine, forming a guanidinium cation. The dimethylamine nitrogen now has a free lone pair available for nucleophilic attack at the electrophilic nitrile carbon. A proton transfer gives the final metformin HCl salt. Having broken down metformin into these two precursors, the challenge now becomes preparing each of these from simple hydrocarbons.
Synthesis of dimethylamine from hydrocarbon
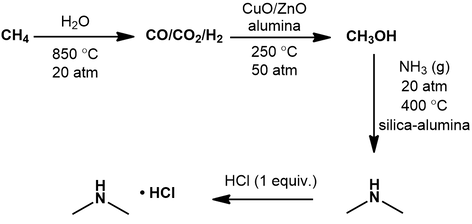
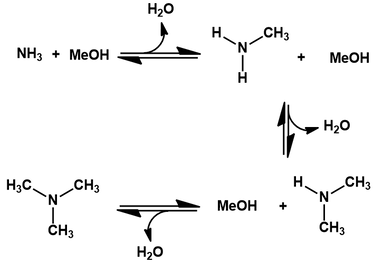
To prepare dimethylamine, we first make methanol. This can be manufactured from various hydrocarbon sources, like natural gas, coal, or even biomass. To begin, the carbon source is transformed into a gas mixture called “synthesis gas” by steam reforming [3]. This involves reacting it with water at high temperature and pressure, producing a mixture of carbon monoxide, carbon dioxide and hydrogen gas. Next, the hydrogenation of CO and CO2 is promoted using a CuO/ZnO/alumina catalyst, yielding methanol [4]. This is then converted to dimethylamine by dehydration with ammonia gas under high pressure and temperature [5]. Various catalysts can be used to promote this chemistry, but silica-alumina is currently preferred industrially. In addition to the desired dimethylamine, monomethyl and trimethylamine are also made from this process:
The equilibrium ratio of these species depends on the molar ratio of methanol to ammonia used. Lastly, treatment of dimethylamine with a stoichiometric amount of hydrochloric acid affords the hydrochloride salt.
Synthesis of cyanoguanidine from hydrocarbon
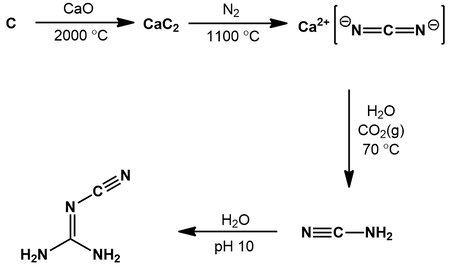
Our synthesis of cyanoguanidine begins with a source of elemental carbon: coke. This is made by heating hydrocarbon-rich materials like oil or coal in the absence of oxygen. Calcium carbide (CaC2) is prepared by heating a mixture of coke and calcium oxide (CaO) to a remarkably hot 2000 °C in an electric arc furnace [6]. Carbon monoxide is evolved as a by-product.
Next, the calcium carbide is converted to calcium cyanamide (CaCN2) by reaction with N2 gas at 1100 °C (the Frank-Caro process) [7]. Finally, calcium cyanamide is transformed to the desired cyanoguanidine over a two step process [8]. First, the calcium cyanamide is reacted with water in the presence of carbon dioxide:
Next, the calcium carbide is converted to calcium cyanamide (CaCN2) by reaction with N2 gas at 1100 °C (the Frank-Caro process) [7]. Finally, calcium cyanamide is transformed to the desired cyanoguanidine over a two step process [8]. First, the calcium cyanamide is reacted with water in the presence of carbon dioxide:
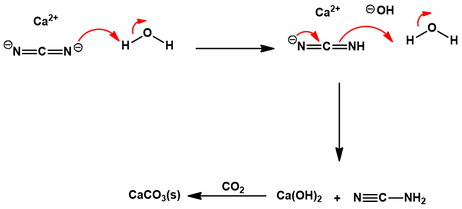
The calcium cyanamide is protonated twice by water, generating cyanamide and calcium hydroxide. The calcium hydroxide is “trapped” by reaction with carbon dioxide, giving insoluble calcium carbonate as a by-product. The water is then basified (pH 10), promoting the dimerization of cyanamide to cyanoguanidine:
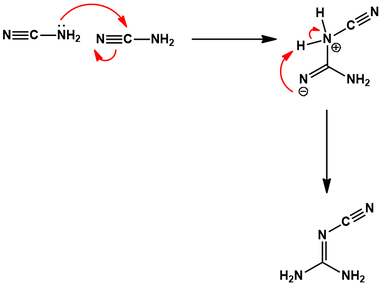
Basic conditions ensure the amine of cyanoguanidine is deprotonated. Thus, the amine of one cyanamide can do a nucleophilic attack on the nitrile carbon of another. The resulting intermediate undergoes a proton transfer, giving the product cyanoguanidine.
Production of reagents: ammonia and calcium oxide
Continuing the theme of industrial chemicals’ origins, it is also worth considering the reagents required for this synthesis. In our preparation of dimethylamine, ammonia (NH4) was needed. This is obtained from hydrogen and nitrogen gas via the Haber process. We’ve seen that hydrogen can be produced from steam reforming and nitrogen is highly abundant in Earth’s atmosphere (78%).
In addition, our synthesis of cyanoguanidine required calcium oxide (CaO). This is cheaply accessible through thermal decomposition of calcium carbonate:
In addition, our synthesis of cyanoguanidine required calcium oxide (CaO). This is cheaply accessible through thermal decomposition of calcium carbonate:
Calcium carbonate is widely available from natural sources like limestone or seashells.
In summary, a useful and structurally interesting drug, metformin, can be prepared using remarkably simple starting materials: hydrocarbon, water, limestone, and nitrogen.
In summary, a useful and structurally interesting drug, metformin, can be prepared using remarkably simple starting materials: hydrocarbon, water, limestone, and nitrogen.
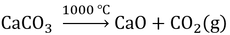
 RSS Feed
RSS Feed