Ki vs Kd
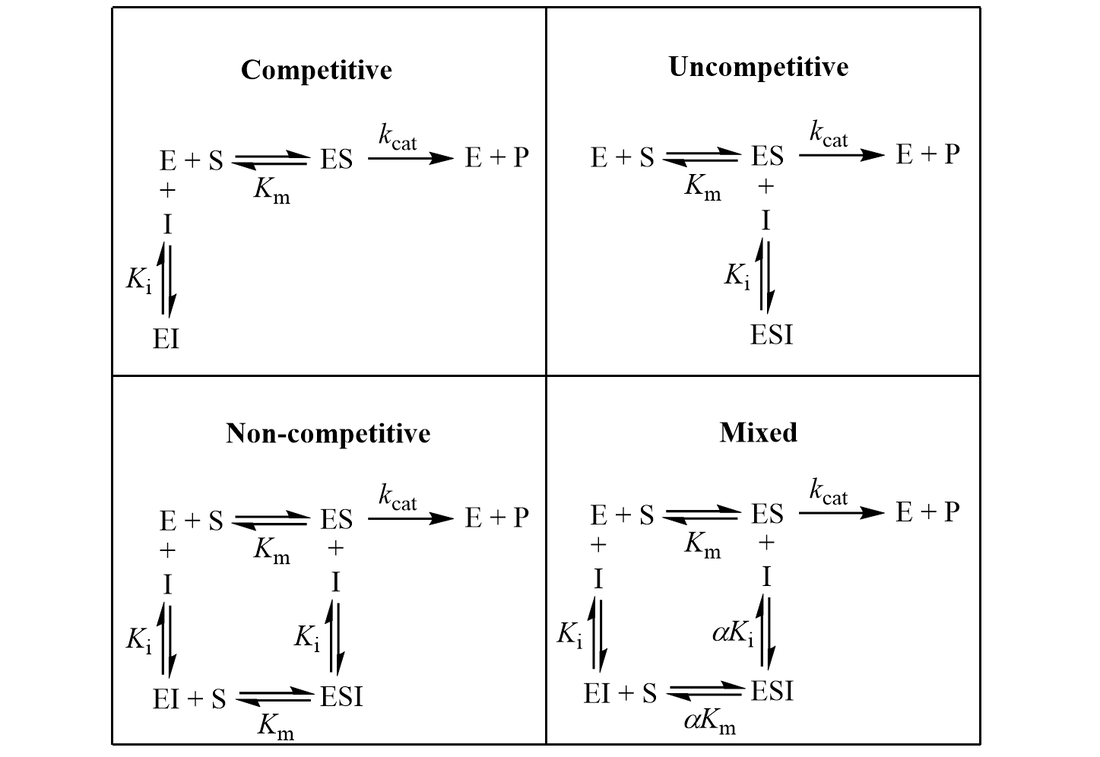
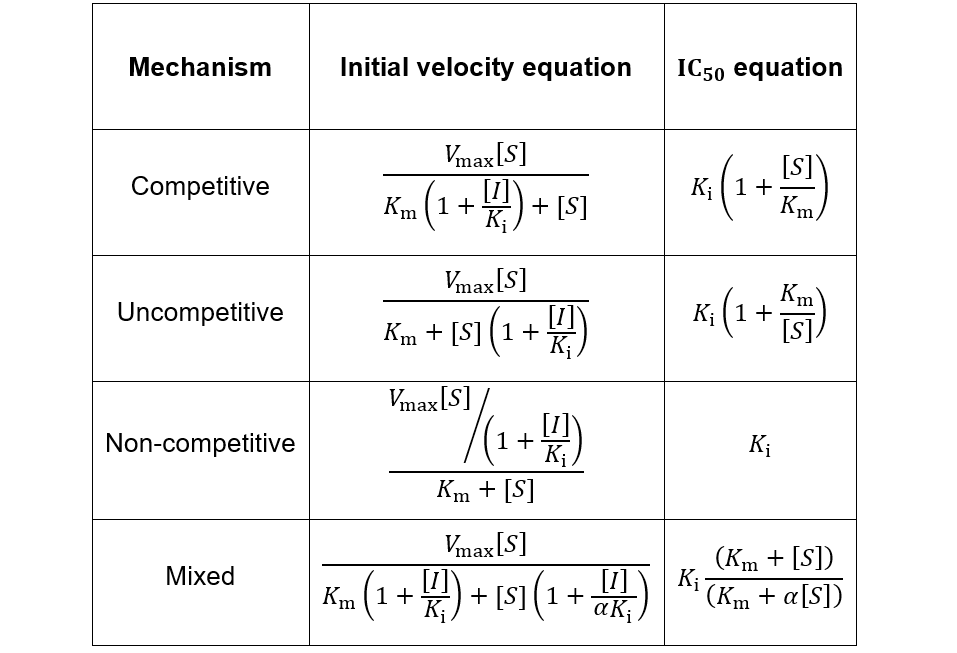
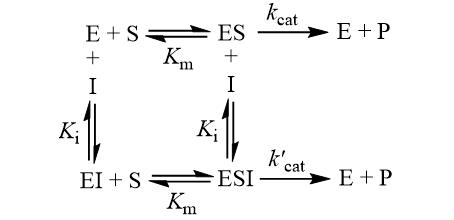
The Ki inhibition constant also represents a dissociation constant, but more narrowly for the binding of an inhibitor to an enzyme. That is, a ligand whose binding reduces the catalytic activity of the enzyme. The binding equilibrium described by the Ki value depends on the kinetic mechanism of inhibition. Common options include competitive, uncompetitive, non-competitive, and mixed inhibition. I define the equilibria for each below.
Importantly, Ki values only accurately report a binding constant when the kinetic mechanism is correctly identified. The term ‘Ki’ is used whenever this binding constant is measured through inhibition kinetics, while ‘Kd’ is preferred when the binding is measured more directly (e.g. by fluorescence quenching, isothermal titration calorimetry, or surface plasmon resonance).
IC50 vs Ki
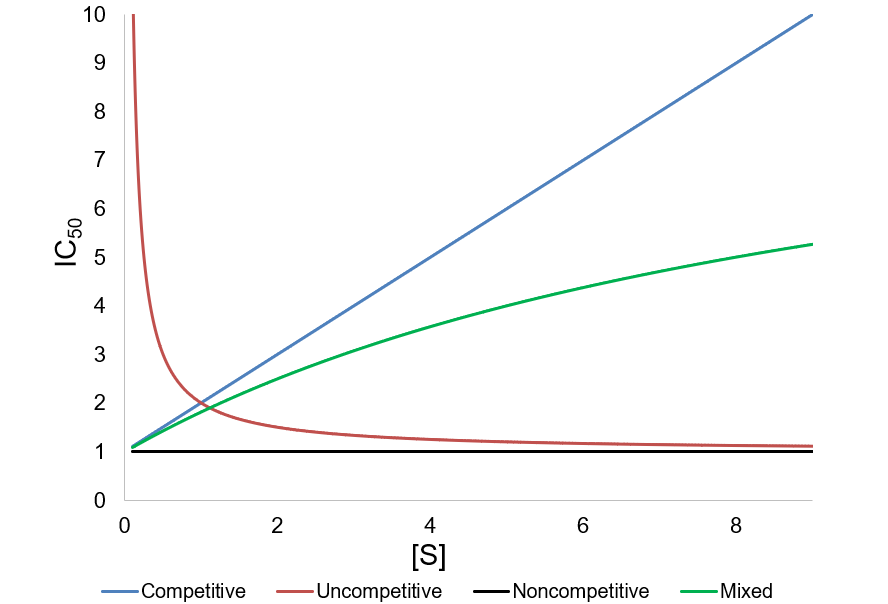
IC50 values are also used for quick comparison of the in vitro activity of enzyme inhibitors. Despite their ease of measurement, this use case carries certain limitations and caveats. Meaningful comparison of IC50 values can be challenging, especially when an inhibitor’s mechanism is unknown or the experimental details are not reported in sufficient detail. IC50 values are highly dependent on the substrate concentration, with the nature of this dependence being determined by the inhibition mechanism. To demonstrate this, I show how IC50 and Ki are related for related for the common inhibition types.
First, I review how IC50 equations are derived from initial velocity equations, taking competitive inhibition as an example. An enzymatic reaction’s initial velocity under competitive inhibition (vi) is given by:
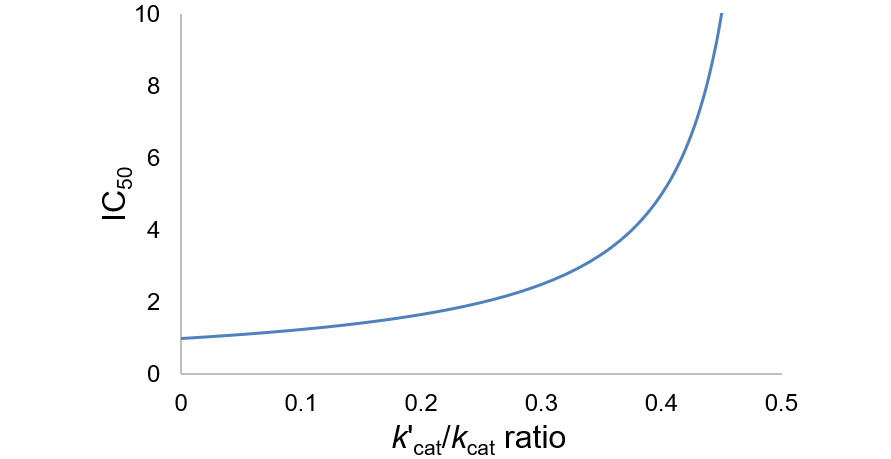
As a final caveat, another complication with IC50 values comes in the case of “tight binding” inhibitors, where the enzyme concentration affects the apparent value. This problem arises when the formation of enzyme-inhibitor complex appreciably depletes the concentration of free inhibitor (specifically, when Ki < [E]T). In these situations, a correction can be applied to account for the effect of this on the IC50 value.
IC50 vs EC50
EC50 values are commonly used for in vivo measurements made on cells or animals; incomplete inhibition of a phenotype is often seen in complex biological systems. In principle though, this nomenclature can also be used for comparing the in vitro activity of partial enzyme inhibitors. As an example, consider a partial non-competitive inhibitor.
In contrast to IC50 values, EC50 reports on the binding affinity of the inhibitor, regardless of its efficacy. The fraction of enzyme (f) bound by inhibitor is given by:
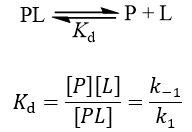
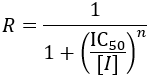
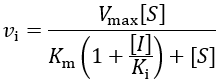
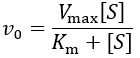
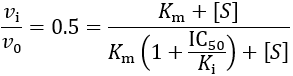
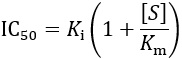
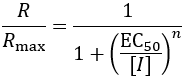
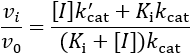
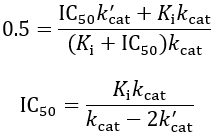
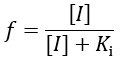
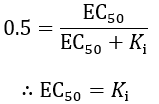
 RSS Feed
RSS Feed