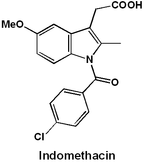
In this article, I explore synthetic routes for the production of indomethacin. Indomethacin (sometimes also called indometacin) is a widely used nonsteroidal anti-inflammatory drug (NSAID). Like other members of this family, it works by inhibiting the cyclooxygenase enzymes (COX-1 and 2), thereby decreasing prostaglandin levels. This is medically useful for decreasing inflammation, fever, and pain. I begin with a retrosynthetic analysis and illustrate a “traditional” synthesis of this compound. Next, I present a more efficient, modern synthesis developed by scientists at Merck. I clarify the mechanisms of all reactions with arrow pushing diagrams.
Retrosynthetic analysis of indomethacin
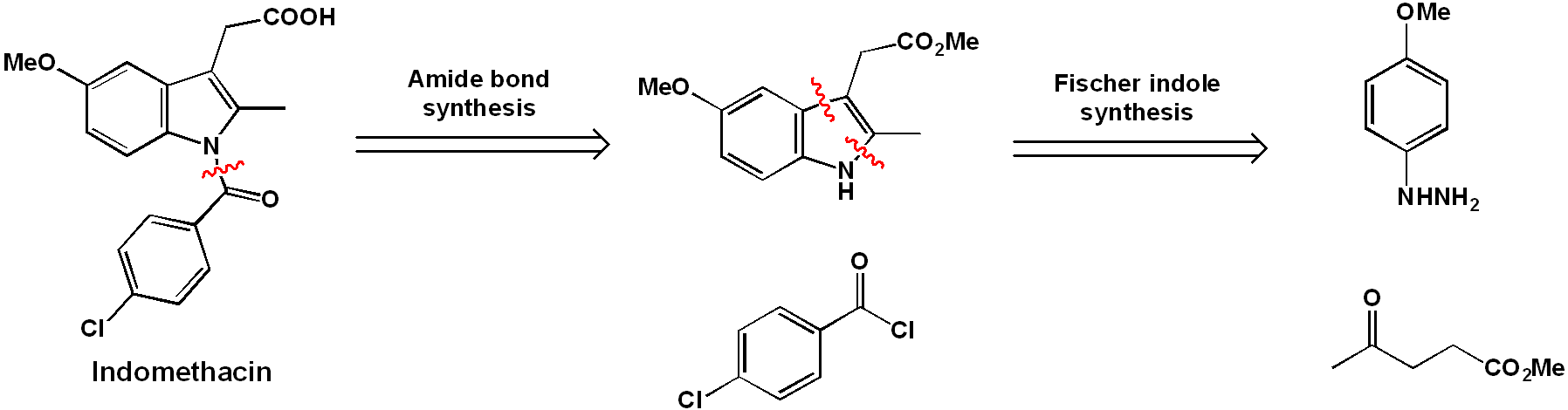
The core structure of the drug is an indole ring, this presumably is the origin of the “indo” in its name. The most important step in the synthesis will be the formation of this aromatic heterocycle. There are many Name reactions devoted to indole synthesis, but one of the oldest, and simplest is the Fischer indole synthesis. The indole N-linked group can be attached after the indole is formed (amide coupling step).
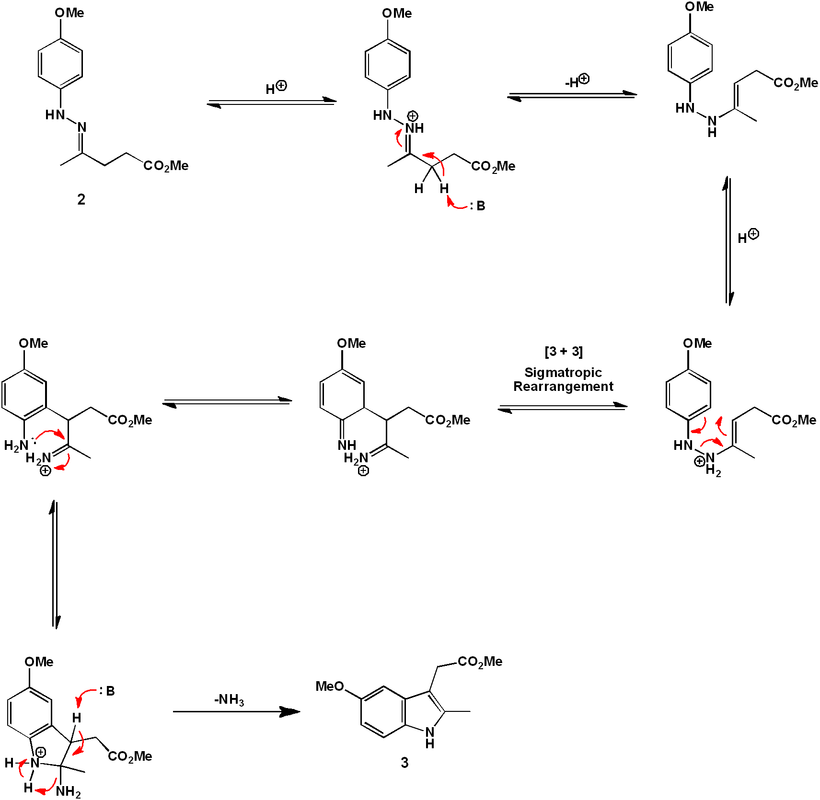
"Traditional" synthesis of indomethacin
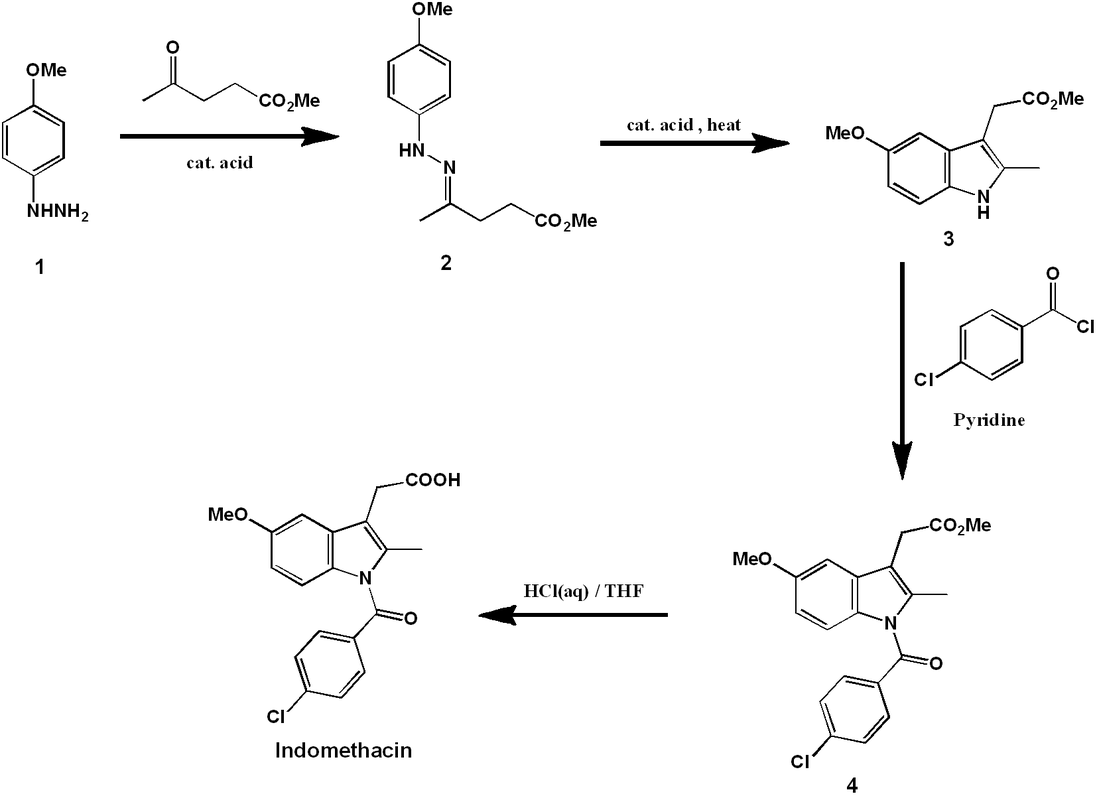
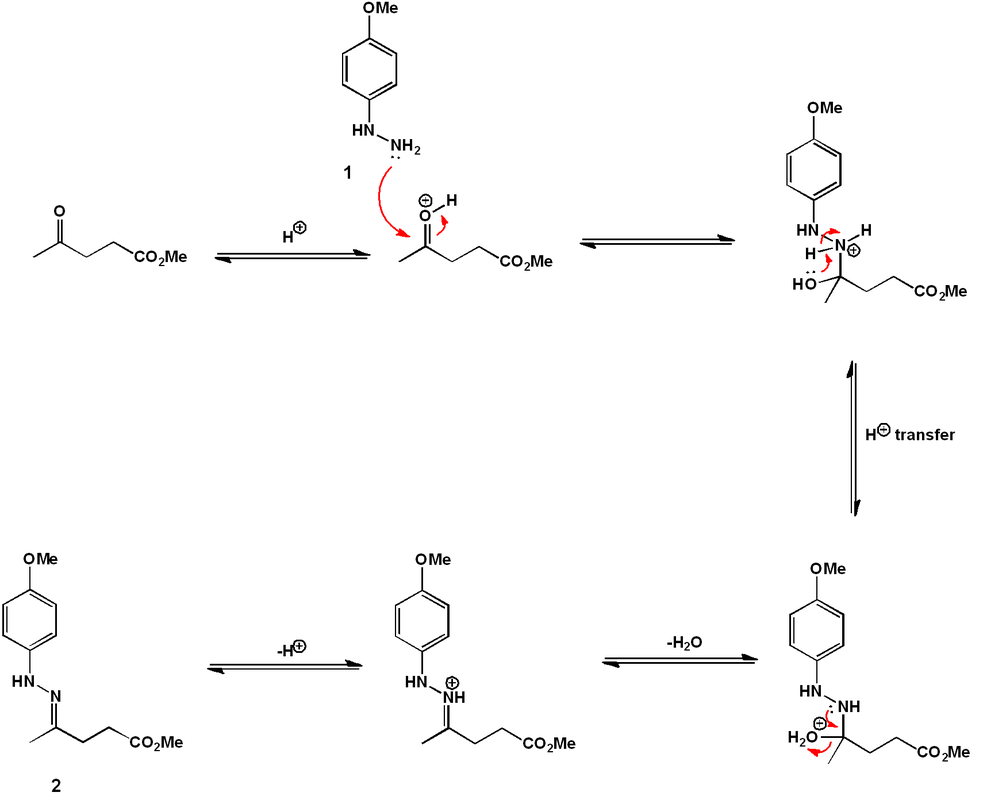
The comprehensive synthetic organic reaction sequence is outlined above. The synthesis begins with 4-methoxyphenyl hydrazine (1). The phenylhydrazone (2) is formed by reaction with methyl levulinate under acid catalyzed-conditions.
Heating of (2) allows isomerization to an enamine and a subsequent cyclic [3,3] sigmatropic rearrangement. Elimination of ammonia gives the indole (3).
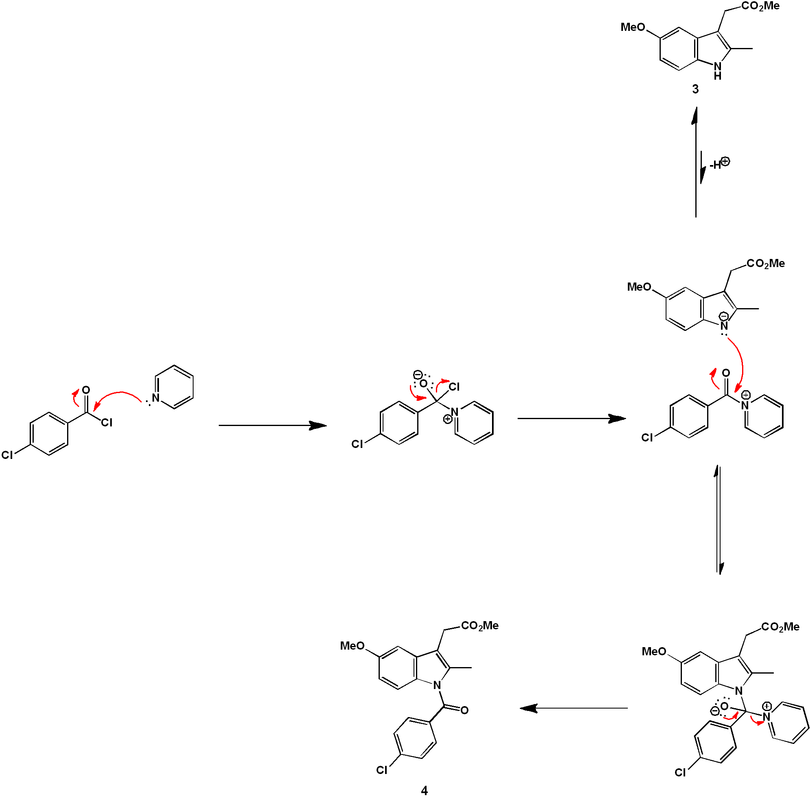
Next, the chlorobenzamide group is coupled to the indole nitrogen of (3) via acylation with 4-chlorobenzoic acid chloride, giving (4). The reaction is carried out in the presence of excess pyridine, which accomplishes two jobs. Firstly, it is a weak base and so can accept the acidic HCl protons released over the course of the reaction, allowing it to go to completion. Furthermore, it is a good nucleophile and so can speed up the reaction by acting as a nucleophilic catalyst.
Compound (4) is nearly identical to the product, except it has a methyl ester instead of a free carboxylic acid group. Acidic hydrolysis of this ester gives the final product, indomethacin.
Modern synthesis of indomethacin
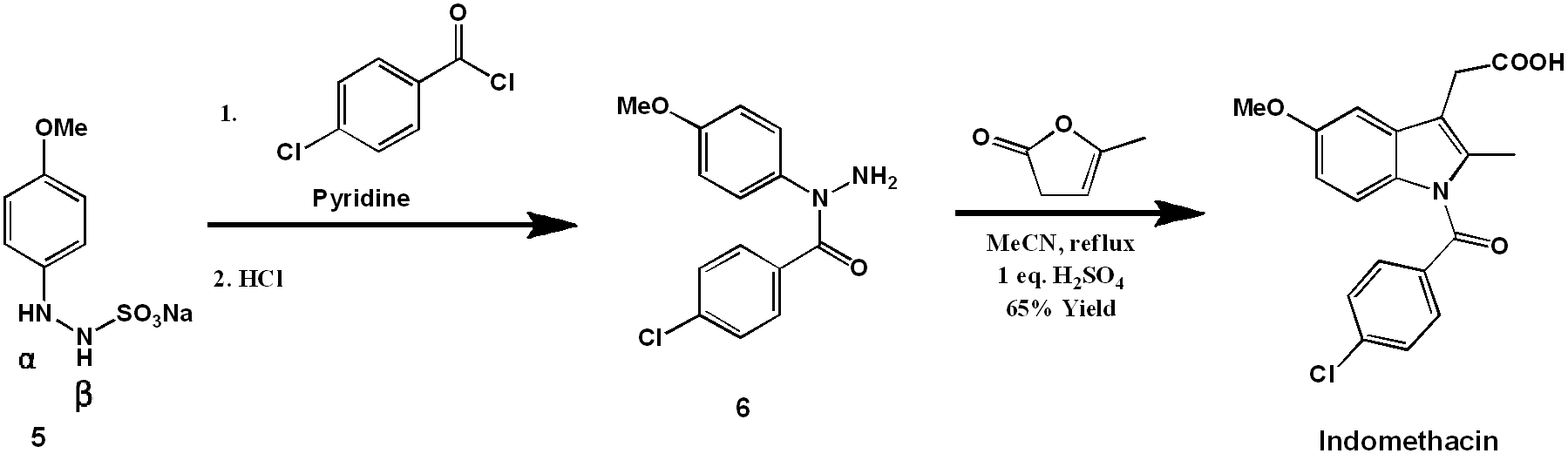
While the previous approach was conceptually simple, the importance of this compound as a pharmaceutical has motivated attempts to reduce the number of steps and improve the overall yield of the synthesis. The result of Merk scientists’ efforts are summarized below, in an elegant two-step synthesis [1].
Here the chlorobenzamide group is coupled onto the molecule before the indole-forming step, eliminating the need for carboxylate protection. The synthesis starts with (5), a β-sulfonate modified aryl hydrazine. Acylation of aryl hydrazines normally occurs preferentially at the β-nitrogen. In contrast, The sulfonate group of (5) is electron-withdrawing, reducing the nucleophilicity of the β-nitrogen and favoring acylation at the α-position [2]. The α-acyl-arylhydrazine (6) can therefore be prepared from (5) by pyridine-catalyzed acylation with 4-chlorobenzoic acid chloride. This process is mechanistically equivalent to the pyridine-catalyzed acylation of the previous synthesis. Afterward, the sulfonate is readily hydrolyzed by treatment with dilute acid.
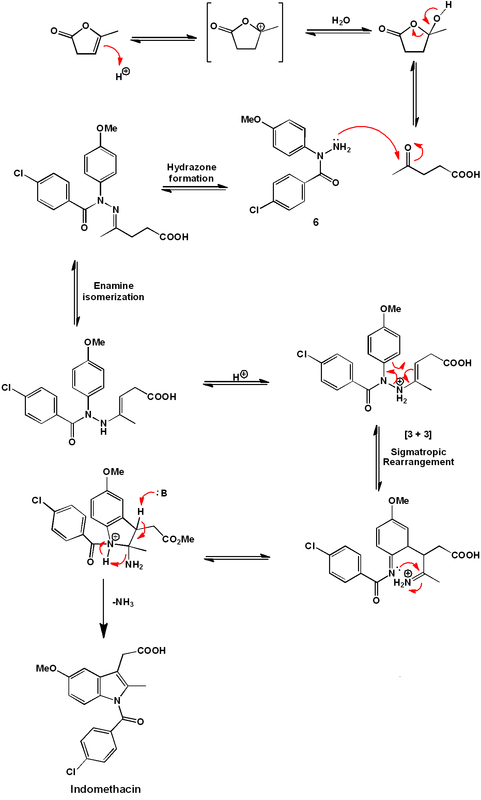
The indole ring is formed by treatment of (6) with α-Angelica lactone under acidic conditions. This dihydropyran functions as a ketone equivalent in the Fischer indole synthesis, as shown mechanistically below. Efficiently, under the conditions developed, the hydrazine formation and the [3 + 3]-sigmatropic rearrangement occur simultaneously under one reaction condition.
The indole ring is formed by treatment of (6) with α-Angelica lactone under acidic conditions. This dihydropyran functions as a ketone equivalent in the Fischer indole synthesis, as shown mechanistically below. Efficiently, under the conditions developed, the hydrazine formation and the [3 + 3]-sigmatropic rearrangement occur simultaneously under one reaction condition.
References
[1] Campos, K. R.; Woo, C. S. W.; Lee, S.; Tillyer, R. D. Org. Lett. 2004, 79-82.
[2] Karady, S.; Ly, M. G.; Pines, S. H.; Chemerda, J. M.; Sletzinger, M. Synthesis 1973, 50-51.
[2] Karady, S.; Ly, M. G.; Pines, S. H.; Chemerda, J. M.; Sletzinger, M. Synthesis 1973, 50-51.
 RSS Feed
RSS Feed